Arrhenius Equation Two Point Form
Arrhenius Equation Two Point Form - This has the form y=mx. However the second t 1/2 takes 5 s. This equation has a vast and important application in determinin… Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration falls from 1.0m to 0.5m. Now here we're going to say that. 721 views 2 years ago general. Web now the two point form of the iranians equation shows how changing the temperature can impact the rate constant which uses the variable que. A) 1.5 x 10 5 kj/mol. Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. When plotted in this manner, the value of.
What is the activation energy? Web the equation is commonly given in the form of an exponential function, k = a exp (− e / rt ), and it predicts that a small increase in reaction temperature will produce. Web the arrhenius equation k a e e a. Web use the 2 point form of the arrhenius equation to calculate k at 80.0 °c. However the second t 1/2 takes 5 s. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. This equation has a vast and important application in determinin… When plotted in this manner, the value of. This has the form y=mx. Now here we're going to say that.
Web two point arrhenius equation. Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a. Web now the two point form of the iranians equation shows how changing the temperature can impact the rate constant which uses the variable que. Alright, so now we have two different equations here for two. The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had noted in 1884 that the van 't hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. Web the arrhenius equation k a e e a. However the second t 1/2 takes 5 s. A) 1.5 x 10 5 kj/mol. Web how to calculate activation energy (ea) with arrhenius equation. In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates.
13.4 The Arrhenius Equation YouTube
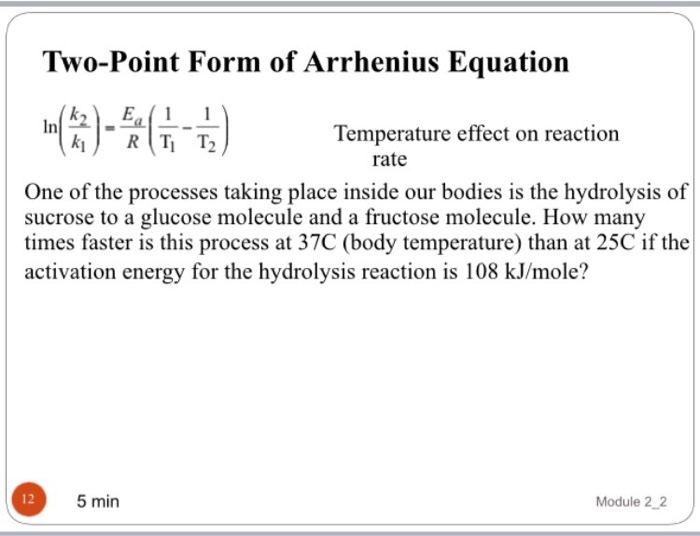
At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can. Alright, so now we have two different equations here for two. The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had.
arrhenius equation Yahoo Image Search Results Energy Activities
At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can. A) 1.5 x 10 5 kj/mol. Web two point arrhenius equation. Web so the natural log of k two is equal to negative ea over r times one over t two this time plus.
Solved TwoPoint Form of Arrhenius Equation In N2.
Alright, so now we have two different equations here for two. Web the arrhenius equation k a e e a. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Web two point arrhenius equation. In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates.
16.2 The Arrhenius equation (HL) YouTube
At two different temperatures t 1 and t 2, the corresponding values of rate constants k 1 and k 2 are known respectively then, we can. A) 1.5 x 10 5 kj/mol. R.t take the natural log ln(k ) ln a e e a. Ln k 2 k 1 = e a r ( 1 t 1 − 1. Web.
Chapter13 chemical
Web now the two point form of the iranians equation shows how changing the temperature can impact the rate constant which uses the variable que. Web two point arrhenius equation. Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. This equation has a vast and important application.
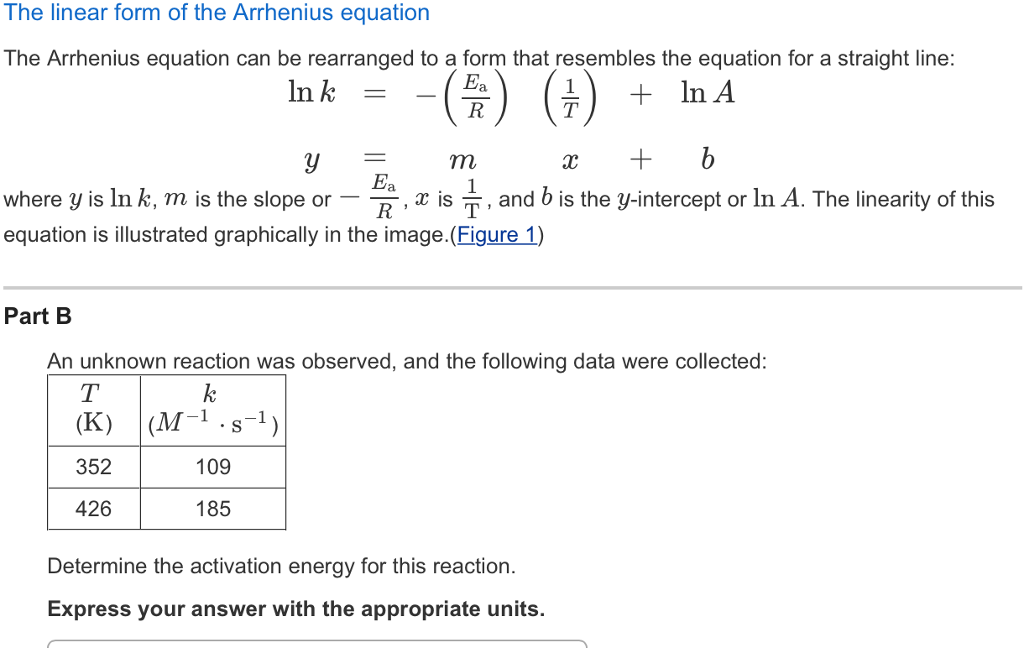
Solved The linear form of the Arrhenius equation The
Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a. This equation has a vast and important application in determinin… Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. Ln.
Two point arrhenius equation, Easy derivation, 3 application
Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. This equation has a vast and important application in determinin… The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had noted in 1884 that the.
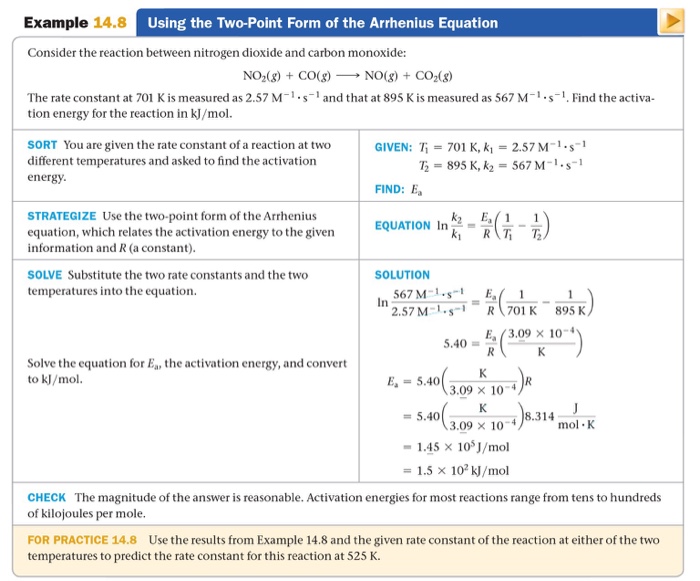
I Need Help With For Practice 14.8. I'm Not Sure H...
This has the form y=mx. Now here we're going to say that. When plotted in this manner, the value of. What is the activation energy? Web two point arrhenius equation.
Chapter13 chemical
In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates. Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a. Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2,.
TwoPoint Form for Arrhenius Equation YouTube
This equation has a vast and important application in determinin… 721 views 2 years ago general. R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Web use the 2 point form of the arrhenius equation to calculate k at 80.0 °c. Web the equation is commonly given in the form of an exponential.
Web Use The 2 Point Form Of The Arrhenius Equation To Calculate K At 80.0 °C.
Web how to calculate activation energy (ea) with arrhenius equation. Web an arrhenius plot plots the log or natural log of the measured parameter (p, d, or s) against the inverse absolute temperature (1/k). Web the arrhenius equation k a e e a. Contrast this with a second order reaction in (b) where during the first 2.5 s t 1/2, the concentration falls from 1.0m to 0.5m.
This Equation Has A Vast And Important Application In Determinin…
R.t expand expression (multiplied inside log so add) ln(k ) ln(a ) ln e e a. Web two point arrhenius equation. Web the arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. Web so the natural log of k two is equal to negative ea over r times one over t two this time plus natural log of a.
When Plotted In This Manner, The Value Of.
In physical chemistry, the arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by svante arrhenius in 1889, based on the work of dutch chemist jacobus henricus van 't hoff who had noted in 1884 that the van 't hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. Alright, so now we have two different equations here for two. What is the activation energy?
This Has The Form Y=Mx.
Now here we're going to say that. A) 1.5 x 10 5 kj/mol. Ln k 2 k 1 = e a r ( 1 t 1 − 1. It uses two (any two points that fall on the line) and the slope of the line (therefore the name.