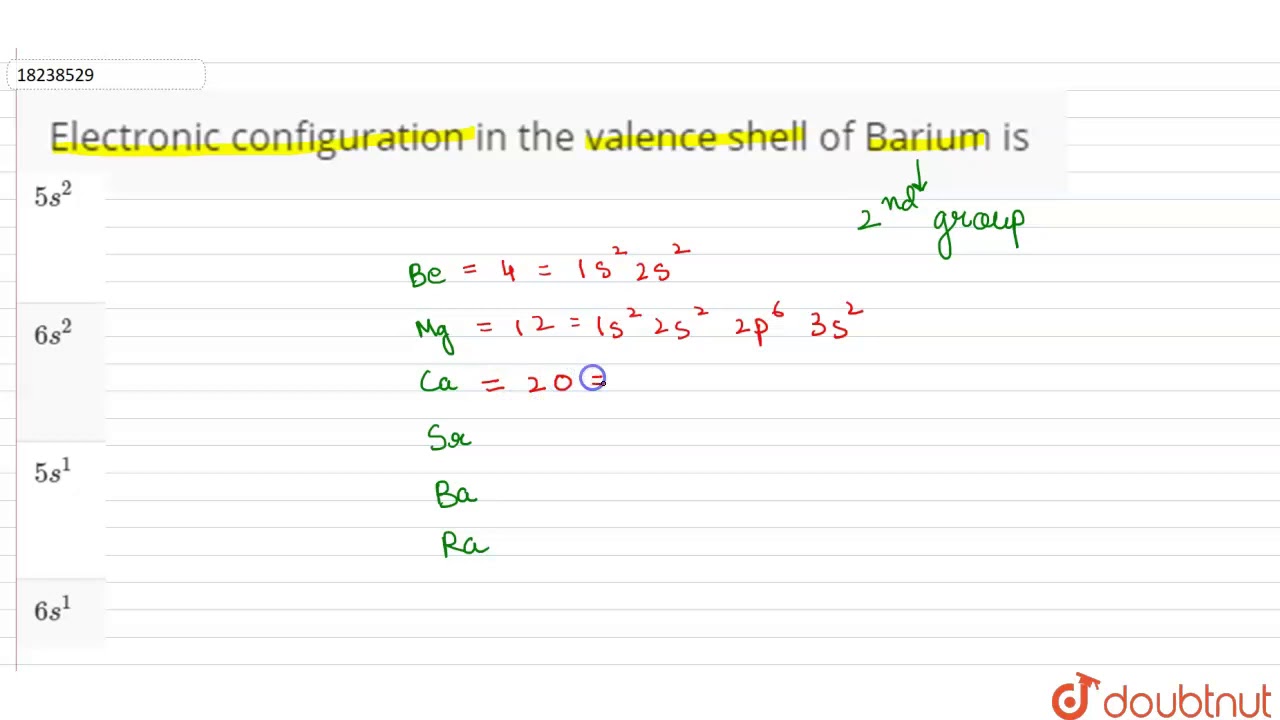
Barium Electron Configuration Long Form
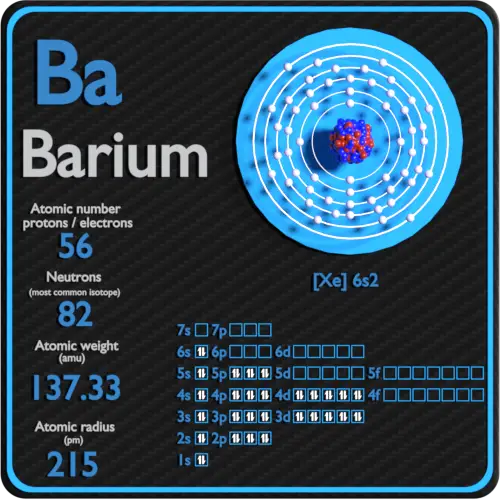
Barium Electron Configuration Long Form - #1 using aufbau principle #2 using periodic table #3 from its bohr model #4 from its orbital diagram. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. 2118 k (1845 °c, 3353 °f) density (near r.t.) 3.51 g/cm 3: In this video we will write the electron configuration for ba 2+, the barium ion. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): Web electron configuration of barium is [xe] 6s2. 1s 2 2s 2 2p 3: The atomic number of barium is 56and it belongs to the sixth period and the second group of the periodic table. 1000 k (727 °c, 1341 °f) boiling point:
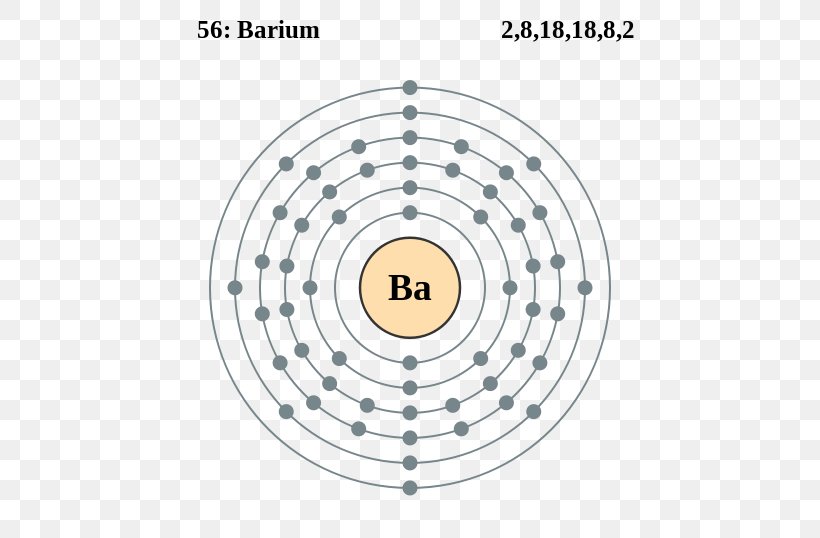
Web electron configuration of beryllium (be) [he] 2s 2: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Electron configuration can be done in two ways. Web barium can be found in the 6th energy level (row) of the periodic table. Web electron configuration of barium. 2, 8, 18, 18, 8, 2: Located in the vi period. Electronic configuration of the barium atom in ascending order of orbital energies: Web electron configurations of the elements (data page) this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web an uncharged barium atom would have 56 electrons also.
Web we can write the electron configuration of barium using four different methods: Electron configuration of oxygen (o. Web how to write the electron configuration for barium. Let’s break down each method in detail. 2, 8, 18, 18, 8, 2: In this video we will write the electron configuration for ba 2+, the barium ion. It is also known as abbreviated electron configuration. Web electron configuration of beryllium (be) [he] 2s 2: Electronic configuration of the barium atom in ascending order of orbital energies: The unabbreviated electron configuration of ba is, 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2.
A stepbystep description of how to write the electron configuration
Electron configuration of oxygen (o. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web how to write the electron configuration for barium. Members of a group typically have similar properties and electron configurations in their outer shell. When liquid (at m.p.) 3.338 g/cm 3 :
Electron Configuration Of Barium cloudshareinfo
25k views 2 years ago. The atomic number of each element increases by one, reading from left to right. Located in the vi period. Number of neutrons (most common/stable nuclide): `1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 4p^6, 4d^10, 5s^2, 5p^6, 6s^2` thus, we can see that s, p.
56. Barium Quicycle Society
Electron configuration can be done in two ways. The chemical symbol of barium is ba. A horizontal row in the periodic table. The 6th row, s block, 2nd column. In this video we will write the electron configuration for ba 2+, the barium ion.
Atomic structure of oxygen stock illustration. Illustration of physics
It is also known as abbreviated electron configuration. The electron configuration of barium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 2, if the electron arrangement is through orbitals. Electron configuration of oxygen (o. Ba (barium) is an element with position number 56 in.
Yeezy Quantum Barium Takes After a Chemical Element & 3 Other Yeezys!
Web barium can be found in the 6th energy level (row) of the periodic table. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: The first two groups (columns) of the periodic table represent the 's' orbital group. We’ll also look at why barium forms a 2+ ion and how the electron. ← electronic configurations of elements.
Symbol and electron diagram for barium Royalty Free Vector
The electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. 1s 2 2s 2 2p 3: Web electron configurations of the elements (data page) this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web we can write the electron configuration of barium using four different methods:.
Barium Periodic Table and Atomic Properties
1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 6 6s 2. Electronic configuration of the barium atom in ascending order of orbital energies: 25k views 2 years ago. Electron configuration of carbon (c) [he] 2s 2 2p 2: Web electron configuration of barium.
Electron Configuration Of Barium cloudshareinfo
It is also in the 2nd group (column) of the periodic table. Electron configuration can be done in two ways. Located in the vi period. Electron configuration of carbon (c) [he] 2s 2 2p 2: Web the electron configuration of ba includes a total of 56 electrons.
Barium electron configuration Newton Desk
The electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. We’ll also look at why barium forms a 2+ ion and how the electron. 2118 k (1845 °c, 3353 °f) density (near r.t.) 3.51 g/cm 3: The number of the principal quantum shell, n, the letter that designates the orbital type (the.
Ba 2+ Electron Configuration (Barium Ion) YouTube
Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and. Electron configuration of carbon (c) [he] 2s 2 2p 2: Let’s break down each method in detail. Web the arrangement of electrons in barium in specific rules in different orbits and orbitals is called the electron.
Number Of Neutrons (Most Common/Stable Nuclide):
Web full electron configuration of barium: 25k views 2 years ago. The chemical element that belongs to the periodic table is called barium, its atomic number is 56 and it is represented by the symbol ba. The atomic number of each element increases by one, reading from left to right.
Electron Configuration Of Carbon (C) [He] 2S 2 2P 2:
In this video we will write the electron configuration for ba 2+, the barium ion. Web a vertical column in the periodic table. Number of electrons (with no charge): Web barium can be found in the 6th energy level (row) of the periodic table.
This Means That The S,P,D,F Electron Configuration For Barium Must End With 6S^2.
Web we can write the electron configuration of barium using four different methods: Web electron configuration of beryllium (be) [he] 2s 2: Electron configuration of boron (b) [he] 2s 2 2p 1: A horizontal row in the periodic table.
1S 2 2S 2 2P 3:
2118 k (1845 °c, 3353 °f) density (near r.t.) 3.51 g/cm 3: Electron configuration of oxygen (o. Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and. The electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2.