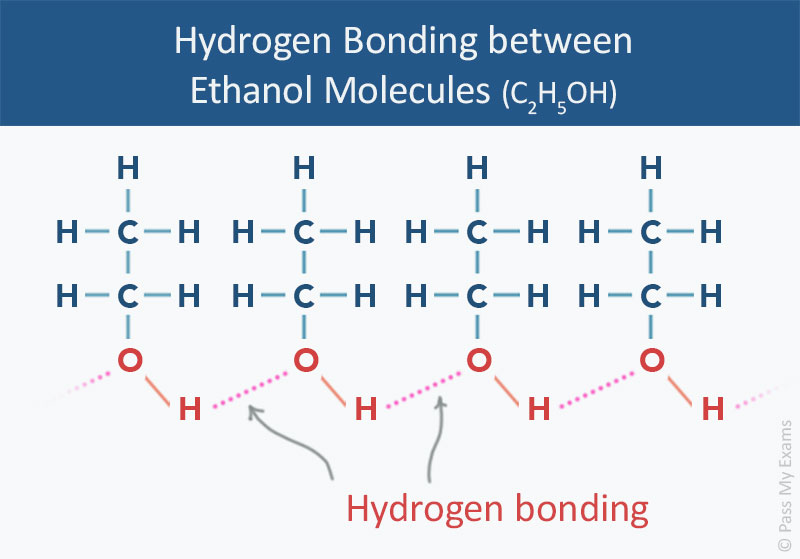
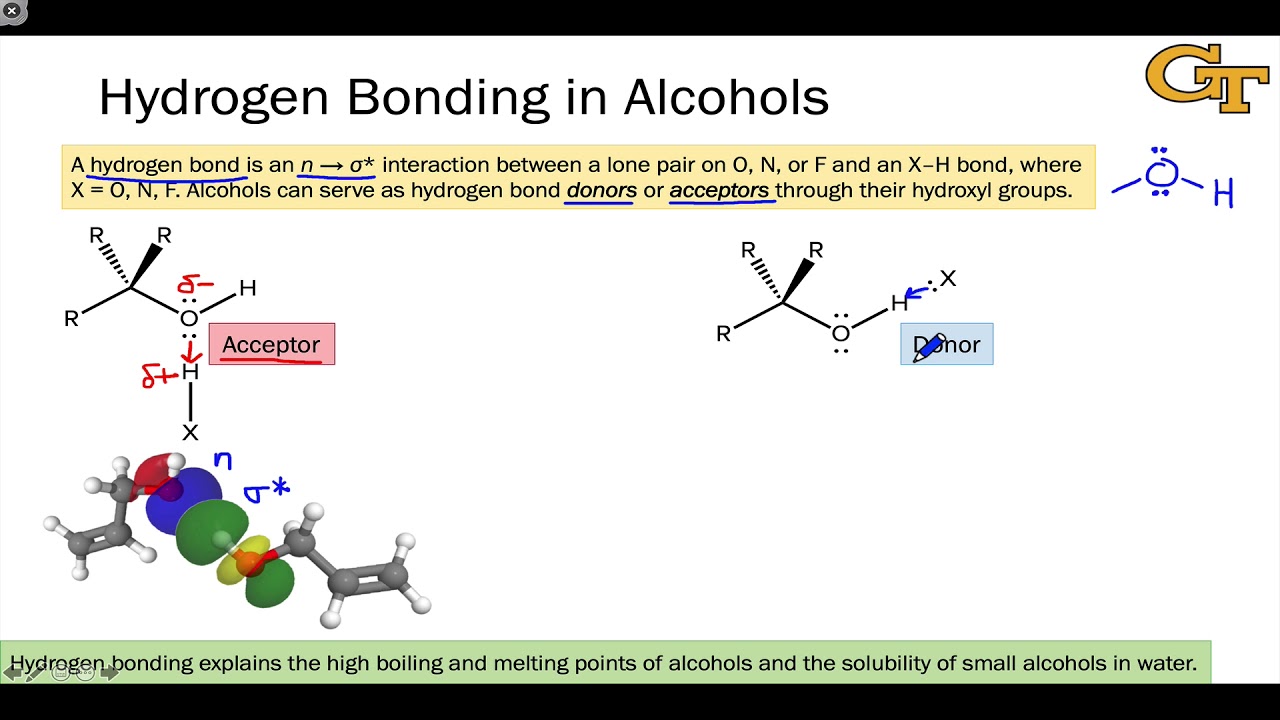
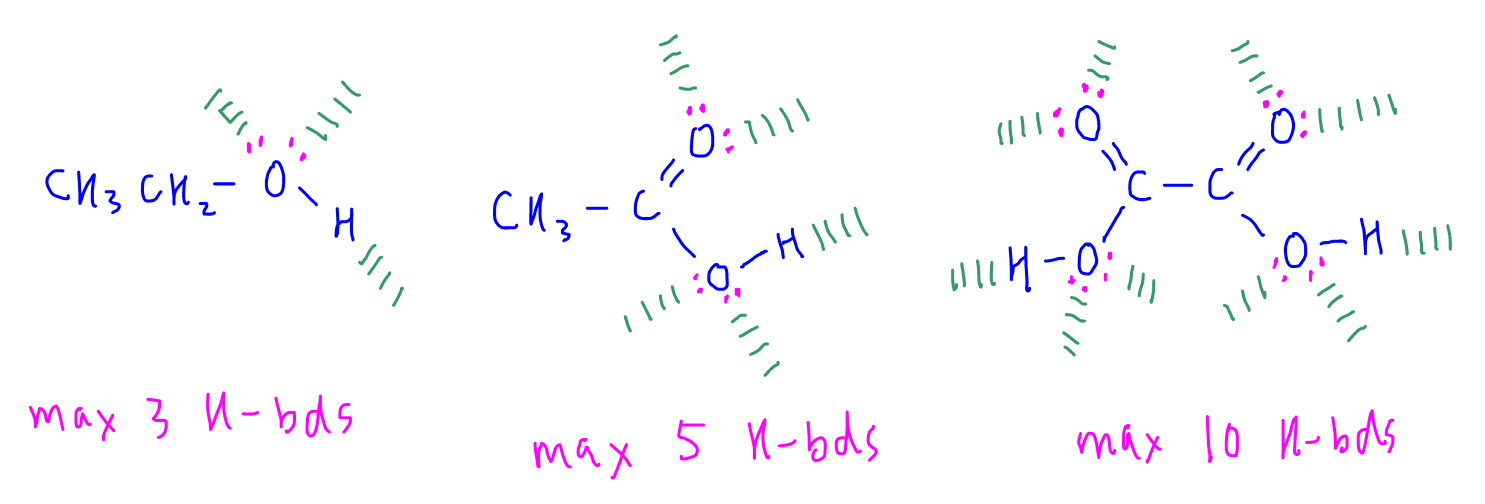Can Ethanol Form Hydrogen Bonds
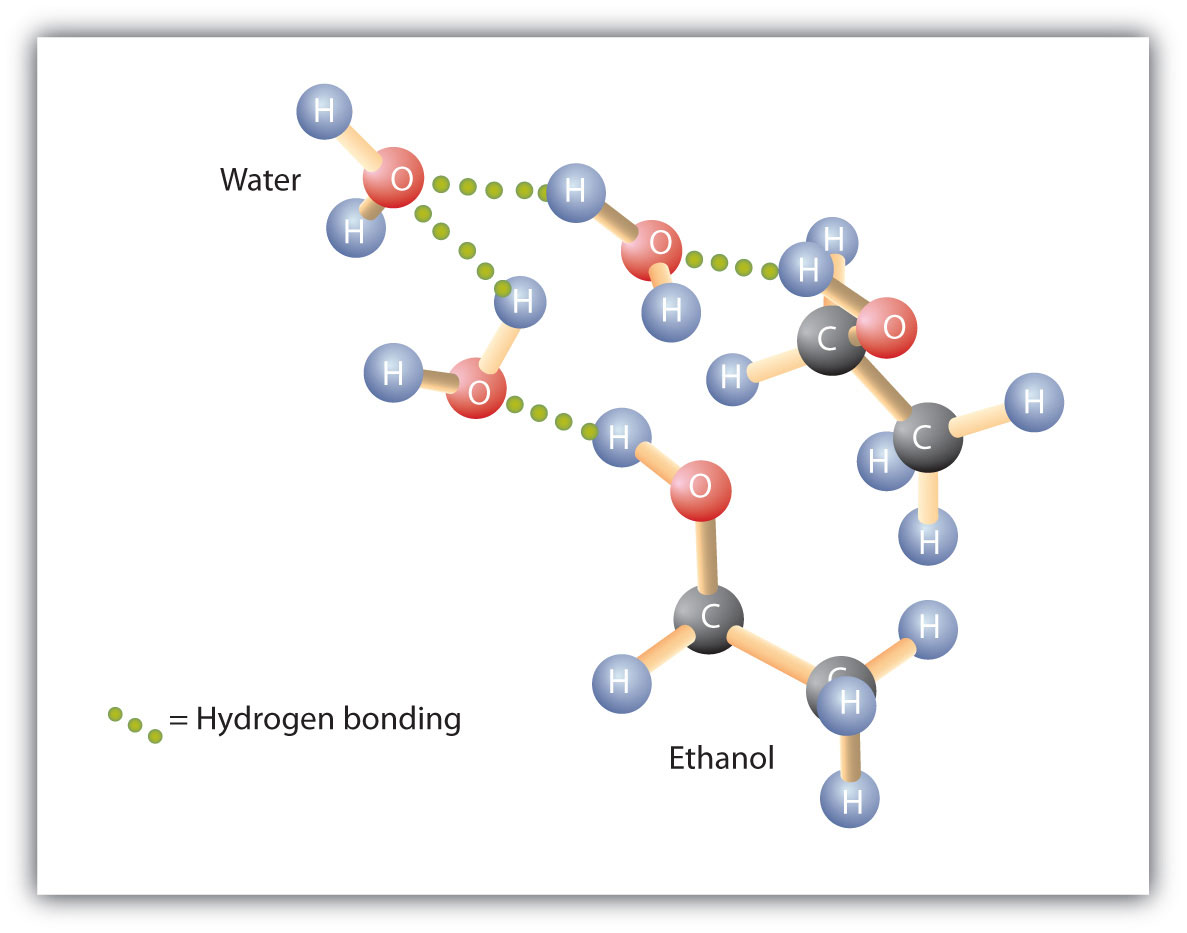
Can Ethanol Form Hydrogen Bonds - This is because the oxygen atom, in addition to forming bonds. The hydrogen bonding is limited by the fact that there is only one hydrogen in. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web because alcohols form hydrogen bonds with water, they tend to be relatively soluble in water. Web can butane, acetone, and ethanol form a hydrogen bond with: Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web ethanol is a longer molecule, and the oxygen atom brings with it an extra 8 electrons. Examples include urea and polyurethane and the natural polymer. Both of these increase the size of the van der waals dispersion forces, and subsequently the. Various molecules may mix and dissolve in each other if they have approximately the same type of polarity.
Examples include urea and polyurethane and the natural polymer. Both of these increase the size of the van der waals dispersion forces, and subsequently the. Hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web can butane, acetone, and ethanol form a hydrogen bond with: Web in an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group, whereas in an ether both hydrogen atoms are replaced by alkyl or aryl groups. Various molecules may mix and dissolve in each other if they have approximately the same type of polarity. Web does ethanol have hydrogen bonding? Polymers that contain carbonyl or amide groups can form hydrogen bonds. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. The hydrogen bonding is limited by the fact that there is only one hydrogen in.
Web because alcohols form hydrogen bonds with water, they tend to be relatively soluble in water. Web does ethanol have hydrogen bonding? This is because the oxygen atom, in addition to forming bonds. Show where the hydrogen bond forms in a lewis structure. Hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web can butane, acetone, and ethanol form a hydrogen bond with: The hydrogen bonding is limited by the fact that there is only one hydrogen in. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web ethanol is able to form hydrogen bonds with the lipids in the bilayer (see hydrogen bonding of alcohol to lipids, below), and these hydrogen bonds reduce the. Both of these increase the size of the van der waals dispersion forces, and subsequently the.
Does Ethanol Form Hydrogen Bonds Printable Form, Templates and Letter
Web does ethanol have hydrogen bonding? Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web can butane, acetone, and ethanol form a hydrogen bond with: Various molecules may mix and dissolve in each other if they have approximately the same type of polarity. In the case of water and.
factorsaffectinghydrogenbond
Web ethanol is able to form hydrogen bonds with the lipids in the bilayer (see hydrogen bonding of alcohol to lipids, below), and these hydrogen bonds reduce the. In the case of water and. Web does ethanol have hydrogen bonding? This is because the oxygen atom, in addition to forming bonds. Web can butane, acetone, and ethanol form a hydrogen.
9 Hydrogen Bond Examples in Real Life StudiousGuy
Various molecules may mix and dissolve in each other if they have approximately the same type of polarity. Examples include urea and polyurethane and the natural polymer. Both of these increase the size of the van der waals dispersion forces, and subsequently the. Web does ethanol have hydrogen bonding? Web in an alcohol one hydrogen atom of a water molecule.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
Both of these increase the size of the van der waals dispersion forces, and subsequently the. This is because the oxygen atom, in addition to forming bonds. Examples include urea and polyurethane and the natural polymer. In the case of water and. Polymers that contain carbonyl or amide groups can form hydrogen bonds.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
Hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web ethanol is able to form hydrogen bonds with the lipids in the bilayer (see hydrogen bonding of alcohol to lipids, below), and these hydrogen bonds reduce the. Both of these increase the size of the van der waals dispersion forces, and subsequently the. In the.
Does Ethanol Form Hydrogen Bonds Printable Form, Templates and Letter
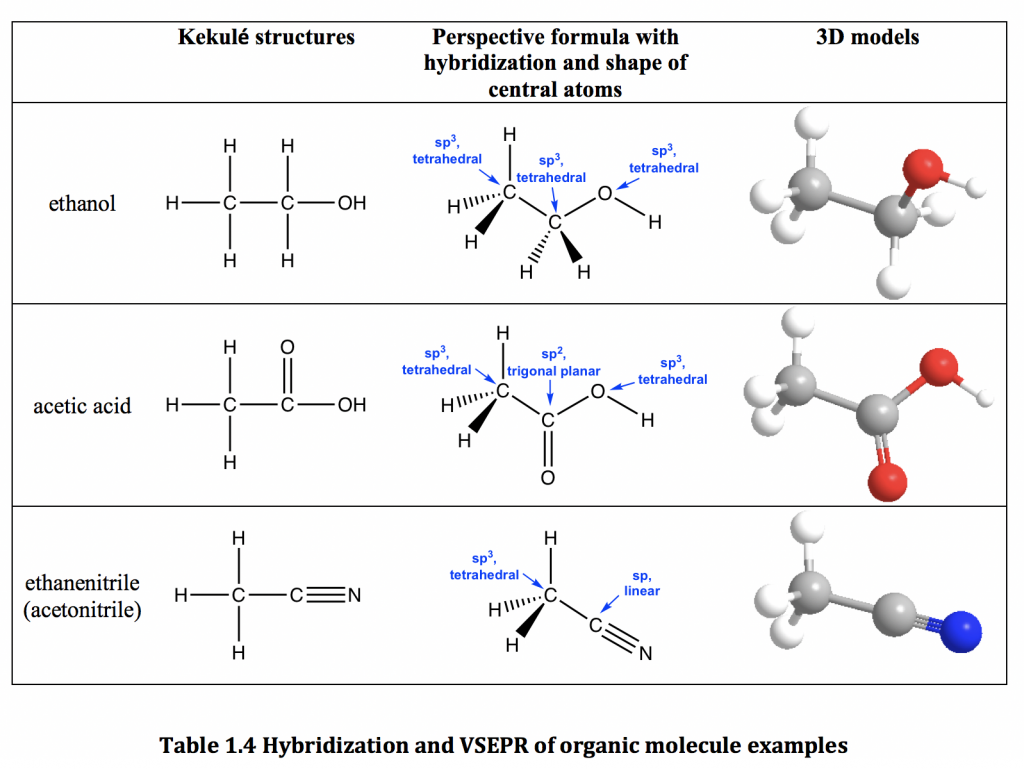
Web in an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group, whereas in an ether both hydrogen atoms are replaced by alkyl or aryl groups. This is because the oxygen atom, in addition to forming bonds. Web does ethanol have hydrogen bonding? Both of these increase the size of the van der waals dispersion.
Physical Properties of Alcohols Easy exam revision notes for GSCE
Web does ethanol have hydrogen bonding? Examples include urea and polyurethane and the natural polymer. Web in an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group, whereas in an ether both hydrogen atoms are replaced by alkyl or aryl groups. Polymers that contain carbonyl or amide groups can form hydrogen bonds. Both of these.
How alcohol affects the human body Chuba Oyolu's Portfolio
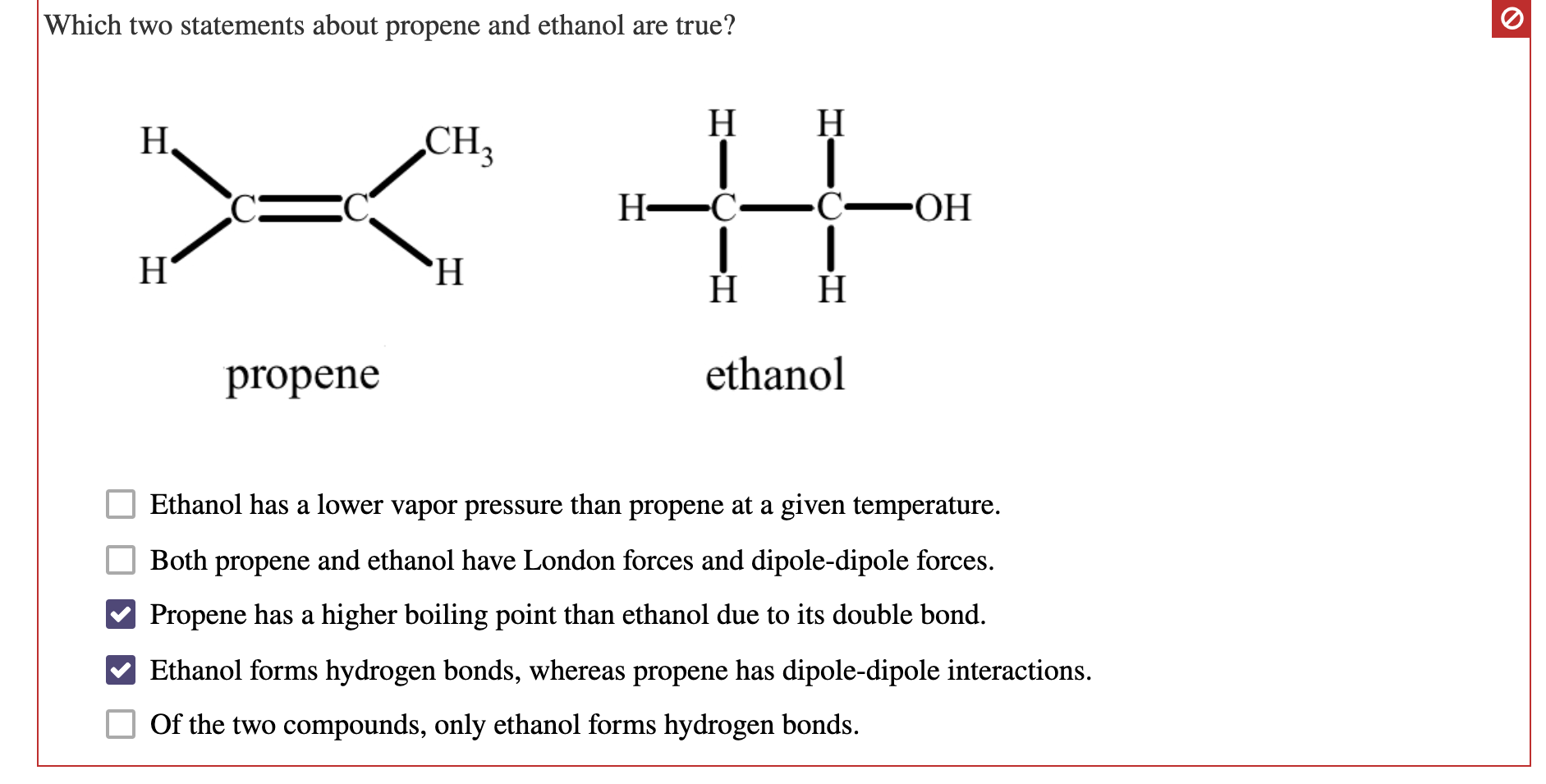
Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web because alcohols form hydrogen bonds with water, they tend to be relatively soluble in water. Web ethanol is a longer molecule, and the oxygen atom brings with it an extra.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
Both of these increase the size of the van der waals dispersion forces, and subsequently the. Examples include urea and polyurethane and the natural polymer. Web because alcohols form hydrogen bonds with water, they tend to be relatively soluble in water. Web in an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group, whereas in.
IGCSE Physical and Chemical Properties of Hydrocarbons IGCSE And IAL
In the case of water and. Web ethanol is able to form hydrogen bonds with the lipids in the bilayer (see hydrogen bonding of alcohol to lipids, below), and these hydrogen bonds reduce the. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web does ethanol have hydrogen bonding? Web can butane, acetone, and.
Web Hydrogen Bonding Can Occur Between Ethanol Molecules, Although Not As Effectively As In Water.
This is because the oxygen atom, in addition to forming bonds. The hydrogen bonding is limited by the fact that there is only one hydrogen in. Polymers that contain carbonyl or amide groups can form hydrogen bonds. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent.
Hydrogen Bonding Can Occur Between Ethanol Molecules, Although Not As Effectively As In Water.
Both of these increase the size of the van der waals dispersion forces, and subsequently the. Web ethanol is a longer molecule, and the oxygen atom brings with it an extra 8 electrons. Web ethanol is able to form hydrogen bonds with the lipids in the bilayer (see hydrogen bonding of alcohol to lipids, below), and these hydrogen bonds reduce the. Show where the hydrogen bond forms in a lewis structure.
Examples Include Urea And Polyurethane And The Natural Polymer.
Web can butane, acetone, and ethanol form a hydrogen bond with: In the case of water and. Web does ethanol have hydrogen bonding? Web in an alcohol one hydrogen atom of a water molecule is replaced by an alkyl group, whereas in an ether both hydrogen atoms are replaced by alkyl or aryl groups.
Web Because Alcohols Form Hydrogen Bonds With Water, They Tend To Be Relatively Soluble In Water.
Various molecules may mix and dissolve in each other if they have approximately the same type of polarity.