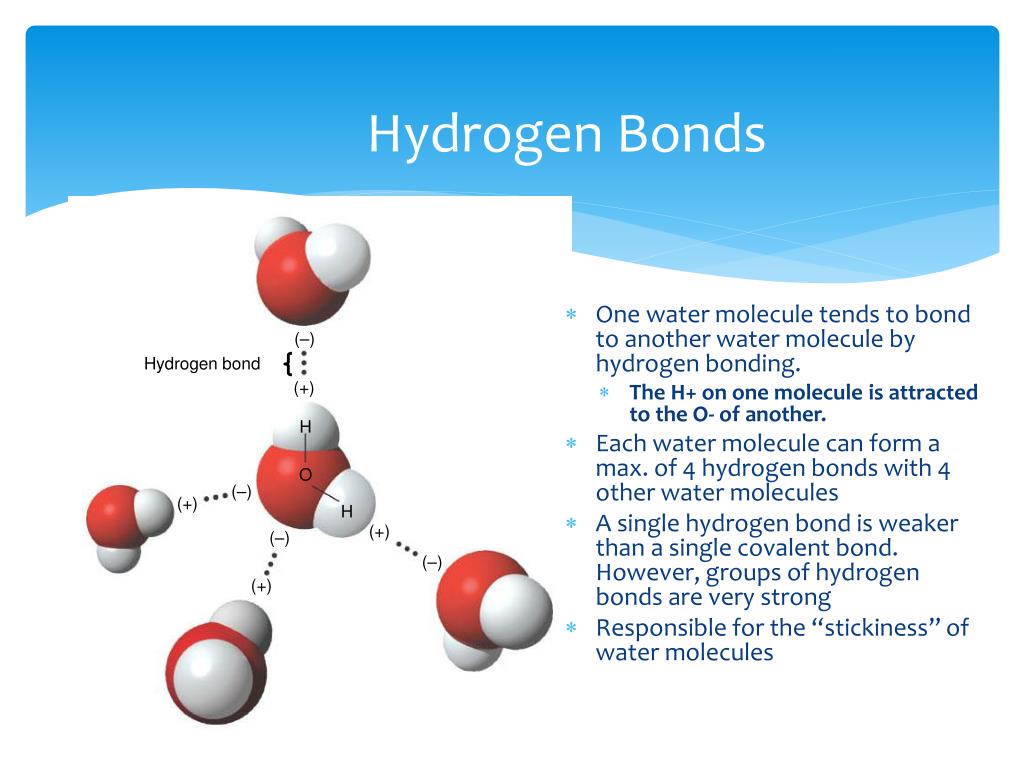
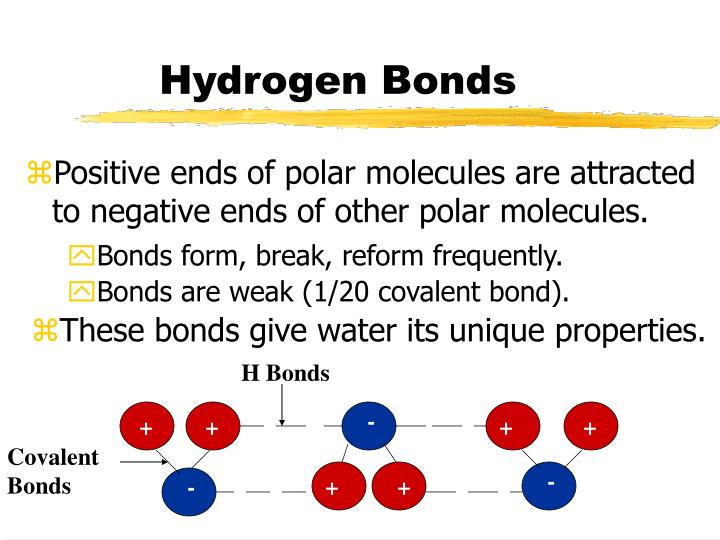
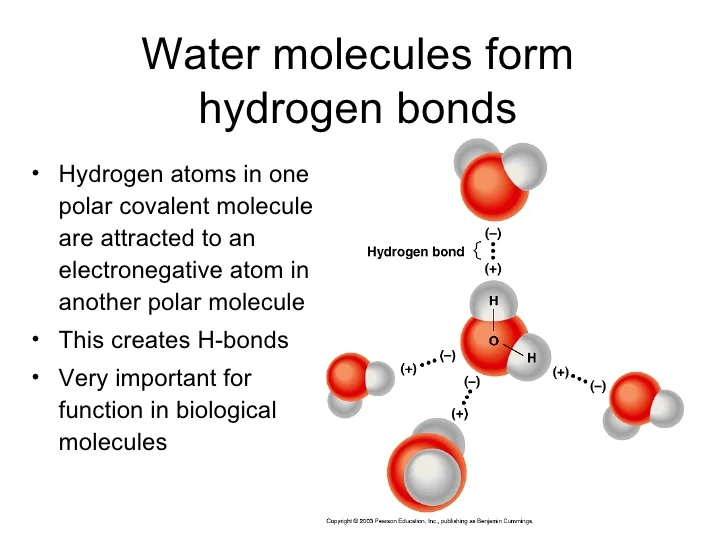
Can Polar Molecules Form Hydrogen Bonds
Can Polar Molecules Form Hydrogen Bonds - Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. Hydrogen bonding is a special type of dipole force between highly polarized molecules. Hence it makes a strong hydrogen bond. Web answer (1 of 4): What is the difference between hydrogen bonds and polar bonds? The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. A simple example of hydrogen bonding can be seen. Hydrogen bonds are intermolecular forces rather than forces within a molecule. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. The most common species for x.
Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. Web hydrogen bonding is explained as the intermolecular forces between polar molecules. Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. The most common species for x. This means the molecules will be soluble in a polar solvent such. A simple example of hydrogen bonding can be seen. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. No , they are different.
Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. When polar molecules are near each. Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. Web because the hydrogen bond occurs between polar regions of a molecule, it is, like all polar attractions, relatively weak. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Hence it makes a strong hydrogen bond. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. A simple example of hydrogen bonding can be seen.
11 Types of scientific changes with examples
No , they are different. Hydrogen bonding is a special type of dipole force between highly polarized molecules. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. Web thus far we have considered only interactions between polar molecules,.
Learn for free about math, art, computer programming, economics
Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. No , they are different. The most common species for x. When polar molecules are near each. Hydrogen bonding is a special type of dipole force between highly polarized molecules.
PPT Properties of Water PowerPoint Presentation, free download ID
Hydrogen bonding is a special type of dipole force between highly polarized molecules. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Web answer (1 of 4): This means the molecules will be soluble in a polar solvent such. A simple example of hydrogen bonding can be seen.
How Do Polar Molecules Form Hydrogen Bonds? Sciencing
A simple example of hydrogen bonding can be seen. Web therefore, it makes the molecules polar. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. Web hydrogen bonding is explained as the intermolecular forces between polar molecules. When polar molecules are near each.
PPT Why Study Chemistry? PowerPoint Presentation ID1433229
Hydrogen bonds are intermolecular forces rather than forces within a molecule. Web hydrogen bonding is explained as the intermolecular forces between polar molecules. Hydrogen bonding is a special type of dipole force between highly polarized molecules. No , they are different. Web nov 19, 2015.
Covalent Bonds Biology for NonMajors I
Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Hydrogen bonding is a special type of dipole force between highly polarized molecules. Web answer (1 of 4): Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. The.
Water Review
No , they are different. This means the molecules will be soluble in a polar solvent such. Web answer (1 of 4): Web hydrogen bonding is explained as the intermolecular forces between polar molecules. Hydrogen bonding is a special type of dipole force between highly polarized molecules.
In hydrogen bonds, do both molecules have to be polar? Quora
Web answer (1 of 4): What is the difference between hydrogen bonds and polar bonds? The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. Web hydrogen bonding is explained as the intermolecular forces.
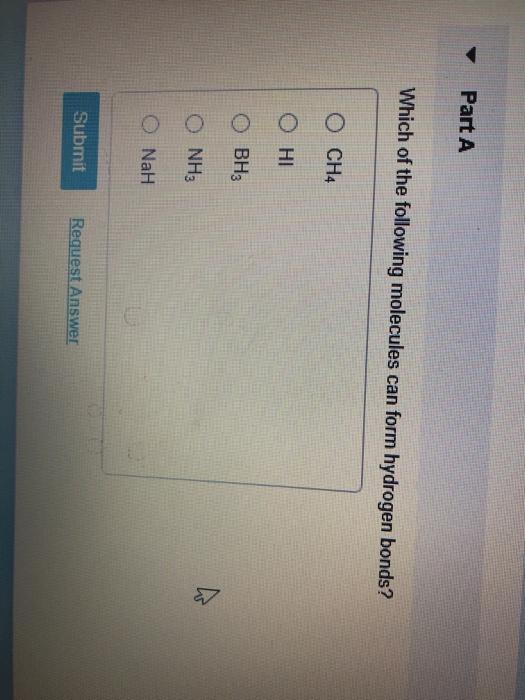
Solved Part A Which of the following molecules can form
Web answer (1 of 4): No , they are different. Hydrogen bonding is a special type of dipole force between highly polarized molecules. Hence it makes a strong hydrogen bond. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds.
Bonds That Hold Water Molecules Together / Intermolecular Forces
The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. Hydrogen bonding is a special type of dipole force between highly polarized molecules. The most common species for x. This means the molecules will be soluble in a polar solvent such. Web answer (1 of 4):
No , They Are Different.
Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. Hydrogen bonding is a special type of dipole force between highly polarized molecules.
Web Hydrogen Bonding Is Explained As The Intermolecular Forces Between Polar Molecules.
The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f. Web answer (1 of 4): A simple example of hydrogen bonding can be seen. Web therefore, it makes the molecules polar.
When Polar Molecules Are Near Each.
Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. Hydrogen bonds are intermolecular forces rather than forces within a molecule. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar.
Web Nov 19, 2015.
Hence it makes a strong hydrogen bond. This means the molecules will be soluble in a polar solvent such. The most common species for x. Web a polar molecule is similar to a magnet, it has a positively charged side and a negatively charged side on the opposite side.