Carbon In Pure Form
Carbon In Pure Form - Web the purest form of carbon is fullerenes since they have a smooth structure without dangling bonds. Carbon dioxide is carbon and oxygen (specifically one carbon molecule and two oxygen molecules) (co2). In the form of anthracite, price per carbon contained, assuming 90% carbon content. Web carbon (from latin carbo 'coal') is a chemical element with the symbol c and atomic number 6. Graphite is black and shiny but soft. It consists of stacked layers of graphene. We often think of coal as being equivalent to carbon, a notion that is reinforced by the common knowledge that diamonds are a result of immense geological pressures and high temperatures acting on coal seams over long periods of time. Web there are a number of pure forms of this element including graphite, diamond, fullerenes and graphene. Diamond is a colourless, transparent, crystalline solid and the hardest known material. Web answer (1 of 5):
It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. A pure form of carbon is only the carbon molecule or an isotope of it (c12 c14). The amount fluctuates by about 20 billion tons over the course of a year and grows by about 5 billion tons every year. Carbon is not very common in pure form, but it’s most common in the form of graphite or diamond. Lavoisier using a giant lens in combustion experiments Web coal and other forms of carbon. Farid bensebaa, in interface science and technology, 2013. Web diamond is a solid form of pure carbon with its atoms arranged in a crystal. The carbon cycle is one of the most important of all biological processes.
Heats, energies, oxidation, reactions, compounds, radii, conductivities models of carbon nanotube structure. Diamond can be found naturally in south africa, brazil, siberia, venezuela, and british guiana (now the republic of guyana). The element symbol for atomic number 6 is c. Web coal and other forms of carbon. It is harder than stainless steel, about as conductive, and as reflective as a polished aluminum mirror. The amount fluctuates by about 20 billion tons over the course of a year and grows by about 5 billion tons every year. Carbon is not very common in pure form, but it’s most common in the form of graphite or diamond. Among coal, anthracite is the coal which is the purest. Solid carbon comes in different forms known as allotropes depending on the type of chemical bond. Web there are three forms of pure carbon:
17 Best images about CK12 Chemistry on Pinterest Freeze, Taste food
Carbon also occurs in a form, discovered only recently, known as fullerenes or buckyballs. The element symbol for atomic number 6 is c. Web the chemical element carbon is classed as a nonmetal. Many other forms of carbon that are commonly found on earth are not pure carbon. Web carbon (from latin carbo 'coal') is a chemical element with the.
graphite a form of pure carbon Rocks and Mineral Specimens for Sale
According to the usgs (united states. Web a happy accident has yielded a new, stable form of pure carbon made from cheap feedstocks, researchers say. The amount fluctuates by about 20 billion tons over the course of a year and grows by about 5 billion tons every year. Web carbon (from latin carbo 'coal') is a chemical element with the.
Carbon Wikipedia
Buckyball carbon holds the promise for opening a whole new field of chemistry (see accompanying sidebar). By far the largest amount of carbon in the atmosphere is in the form of gaseous carbon dioxide. Carbon is found in nature practically everywhere. A pure form of carbon is only the carbon molecule or an isotope of it (c12 c14). Web carbon.
Graphite soft form of pure carbon Stock Photo, Royalty Free Image
Carbon also occurs in a form, discovered only recently, known as fullerenes or buckyballs. It is nonmetallic and tetravalent —its atom making four electrons available to form covalent chemical bonds. Web pure crystalline carbon is found in the form of graphite and diamond. Web diamond is a solid form of pure carbon with its atoms arranged in a crystal. In.
Genius Community Coal
Buckyball carbon holds the promise for opening a whole new field of chemistry (see accompanying sidebar). The amount fluctuates by about 20 billion tons over the course of a year and grows by about 5 billion tons every year. Carbon is found in nature practically everywhere. Heats, energies, oxidation, reactions, compounds, radii, conductivities models of carbon nanotube structure. [14] carbon.
What Is the Difference Between Carbon12 and Carbon14?
Web coal and other forms of carbon. Solid carbon comes in different forms known as allotropes depending on the type of chemical bond. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Web the chemical element carbon is classed as a nonmetal. In diamond, each carbon atom is sp 3 hybridized.
Pure Carbon Black Powder at Rs 60/kilogram Carbon Powder ID
Its discoverer and discovery date are unknown. Among coal, anthracite is the coal which is the purest. In the form of anthracite, price per carbon contained, assuming 90% carbon content. Web answer (1 of 5): Web graphite ( / ˈɡræfaɪt /) is a crystalline form of the element carbon.
GRAPHITE (Native Carbon) from Mexico. As boring as it looks it is a
2.3 activated carbon and carbon nanotube. The amount fluctuates by about 20 billion tons over the course of a year and grows by about 5 billion tons every year. Web carbon is a chemical element, like hydrogen, oxygen, lead or any of the others in the periodic table. Carbon is a very abundant element. Web carbon, chemical element that forms.
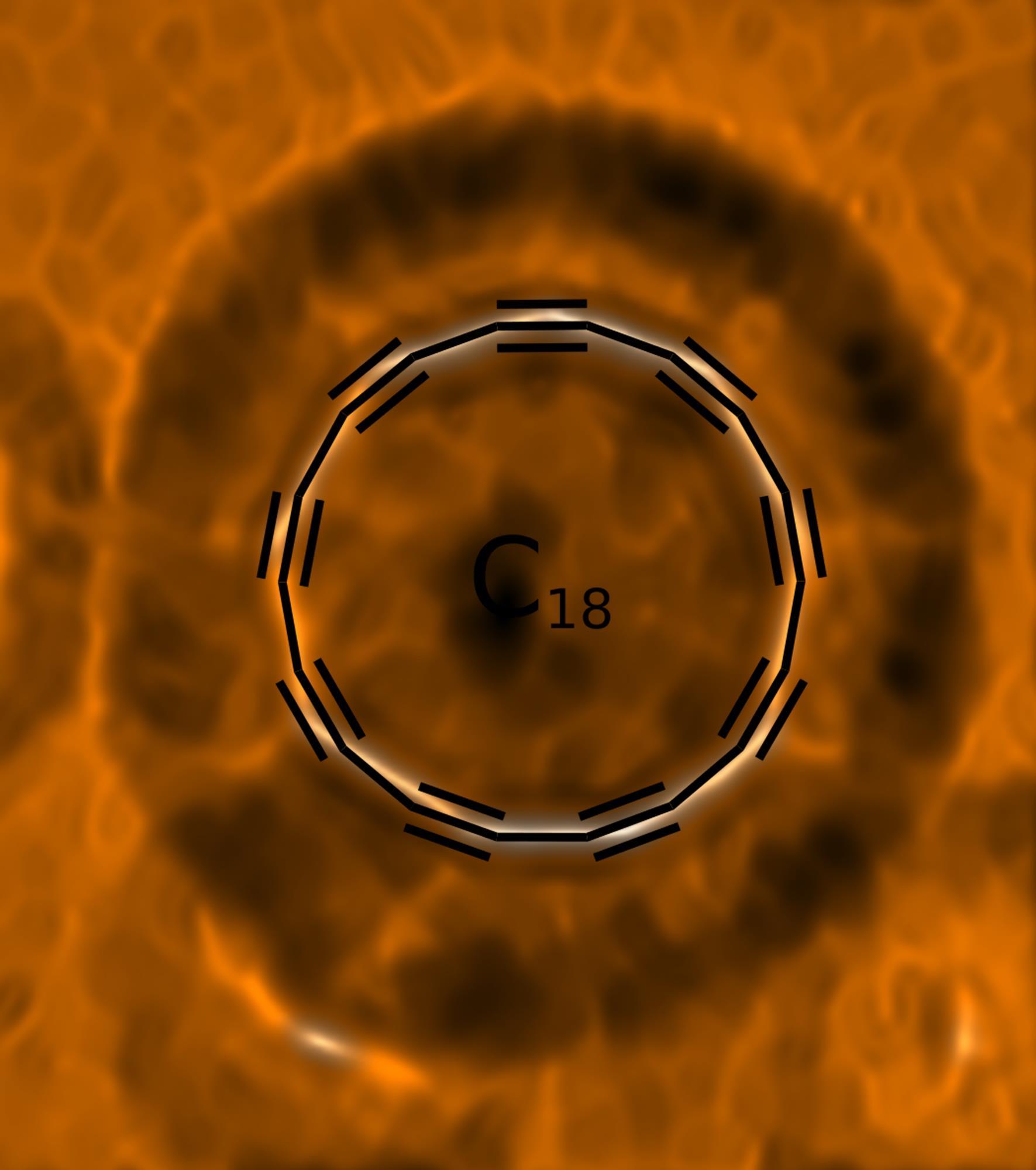
New form of pure carbon made by manipulating atoms Research
Web carbon is a chemical element, like hydrogen, oxygen, lead or any of the others in the periodic table. Many other forms of carbon that are commonly found on earth are not pure carbon. Carbon dioxide is carbon and oxygen (specifically one carbon molecule and two oxygen molecules) (co2). 200 (5.54 × 10 18 kg) 0.122: All three forms are.
Carbon Is A Very Abundant Element.
Web the purest form of carbon is diamond. Solid carbon comes in different forms known as allotropes depending on the type of chemical bond. Many other forms of carbon that are commonly found on earth are not pure carbon. It belongs to group 14 of the periodic table.
Web Carbon Is A Chemical Element, Like Hydrogen, Oxygen, Lead Or Any Of The Others In The Periodic Table.
Web the element name carbon comes from the latin word carbo, which means coal. Web graphite ( / ˈɡræfaɪt /) is a crystalline form of the element carbon. Carbon is widely distributed in coal and in the compounds that make up petroleum, natural gas, and plant and animal tissue. Data zone show more, including:
Primitive Man Used Carbon In The Forms Of Soot And Charcoal.
The amount fluctuates by about 20 billion tons over the course of a year and grows by about 5 billion tons every year. Diamond can be found naturally in south africa, brazil, siberia, venezuela, and british guiana (now the republic of guyana). Graphite occurs naturally and is the most stable form of carbon under standard conditions. Carbon dioxide is carbon and oxygen (specifically one carbon molecule and two oxygen molecules) (co2).
Synthetic And Natural Graphite Are Consumed On Large Scale (300 Kton/Year, In 1989) For Uses In Pencils, Lubricants, And Electrodes.
Its discoverer and discovery date are unknown. It is bonded tetrahedral to four other carbon atoms by strong covalent bonds. Web there are a number of pure forms of this element including graphite, diamond, fullerenes and graphene. Carbon is not very common in pure form, but it’s most common in the form of graphite or diamond.





/Carbon-58ebecc85f9b58ef7e8a1ce4.jpg)