Chapter 17 Thermochemistry Answer Key
Chapter 17 Thermochemistry Answer Key - Study of energy changes that occur during chemical reactions and changes in state. What is chemical potential energy? The energy heats the parts of the engine. Web science chemistry physical chemistry ch 17 thermochemistry practice test 5.0 (1 review) calorie click the card to flip 👆 quantity of heat needed to raise the temperature of 1 g of water by 1c click the. Chapter 17 thermochemistry test answer key. Thermochemistry 17.1 chemical potential energy review questions 1. Write the date at the top of the. Web answer key chapter 17: Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers. Matching (2 points each) write the letter of the correct term with its description.
Study of energy changes that occur during chemical reactions and changes in state. All of the above ____ 7. The energy is transformed into work to move the car. Not all words will be used!!! The property that is useful for keeping track of heat transfers in chemical. Chapter 17 thermochemistry test answer key. Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions: Amount of energy needed to change 1 gram of water 1°c kilocalorie (calorie or food calorie) 2. 11 answer in incomplete combustion; A process that absorbs heat (^h is positive) exothermic.
Write the date at the top of the. All of the above ____ 7. Web heat what is the symbol for heat? A process that absorbs heat (^h is positive) exothermic. The property that is useful for keeping track of heat transfers in chemical. The energy is lost as heat in the exhaust. Web answer key chapter 17: Amount of energy needed to change 1 gram of water 1°c kilocalorie (calorie or food calorie) 2. How is gasoline used as kinetic energy? The amount of heat it takes to raise the temperature of 1g of the substance 1 degree celsius.
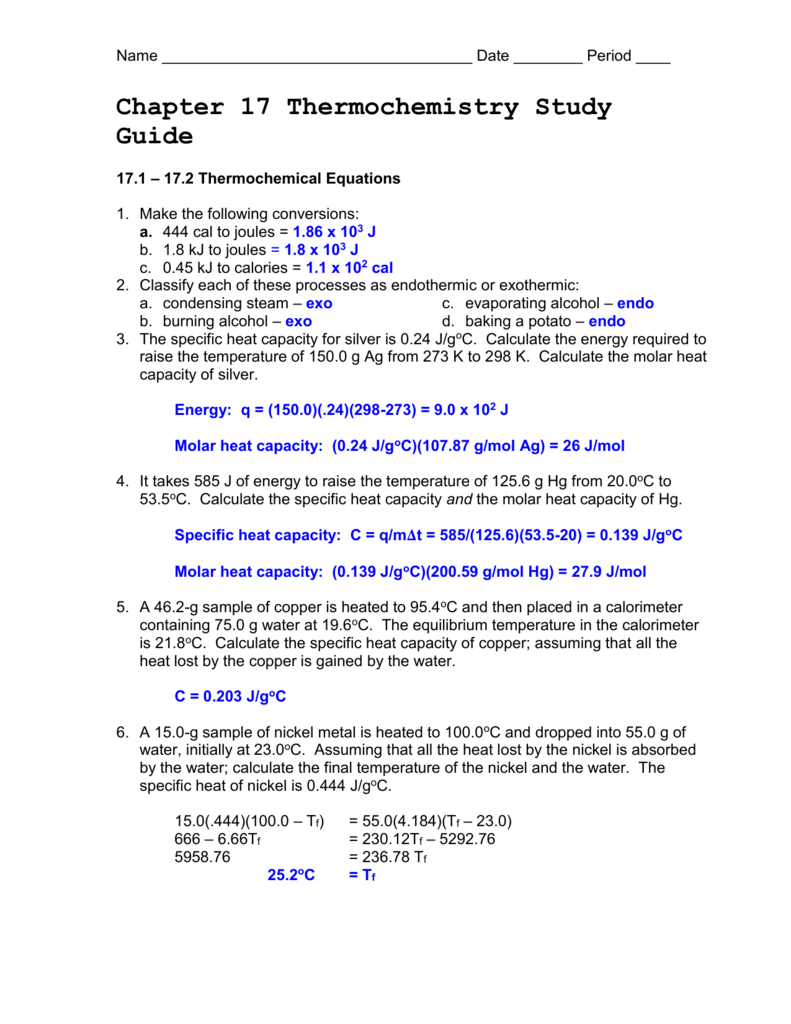
Chapter 17 Thermochemistry Study Guide
Thermochemistry specific heat capacity 1. Energy stored in chemical bonds of a substance. All of the above ____ 7. Chapter 17 thermochemistry test answer key [most popular] 1529 kb/s. Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions:
Chapter 5 Thermochemistry Answers
A piece of metal is heated, then submerged. The energy heats the parts of the engine. Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers. Thermochemistry 17.1 chemical potential energy practice.
PPT Chapter 17 Review “Thermochemistry” PowerPoint Presentation, free
Web this section explains how to construct equations and perform calculations that show enthalpy changes for chemical and physical processes. The energy is lost as heat in the exhaust. A piece of metal is heated, then submerged. The energy heats the parts of the engine. Chapter 17 thermochemistry test answer key.
Chapter 17 thermochemistry sections 17.3 & 17.4
Chapter 17 thermochemistry test answer key. Web this section explains how to construct equations and perform calculations that show enthalpy changes for chemical and physical processes. Web download chapter 17 thermochemistry test answer key: Thermochemistry specific heat capacity 1. The property that is useful for keeping track of heat transfers in chemical.
Chem Basic FB Answer Key Ch 17 (06.14.16)
Thermochemistry 17.1 chemical potential energy practice questions read the material at the link below and answer the questions: The energy heats the parts of the engine. The amount of heat it takes to raise the temperature of 1g of the substance 1 degree celsius. Web the amount of heat needed to increase the temperature of an object exactly 1 degree.
141 Thermochemistry worksheet key Thermochemistry Worksheet Key How
All chemical processes involve the transfer of heat to or from the system. Not all words will be used!!! Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers. Study of energy.
answer key thermochemistry review
Web this section explains how to construct equations and perform calculations that show enthalpy changes for chemical and physical processes. A process that absorbs heat (^h is positive) exothermic. Web heat what is the symbol for heat? Web science chemistry physical chemistry ch 17 thermochemistry practice test 5.0 (1 review) calorie click the card to flip 👆 quantity of heat.
11 Thermochemistry
The energy is transformed into work to move the car. What is chemical potential energy? Energy stored in chemical bonds of a substance. Chapter 17 thermochemistry test answer key [most popular] 1529 kb/s. Not all words will be used!!!
Chapter 17 thermochemistry sections 17.3 & 17.4
11 answer in incomplete combustion; Web download chapter 17 thermochemistry test answer key: The system includes the components involved. Metric unit for heat/energy calorie: How is dynamite used as kinetic energy?
Chapter 17 thermochemistry sections 17.3 & 17.4
How is gasoline used as kinetic energy? A piece of metal is heated, then submerged. The property that is useful for keeping track of heat transfers in chemical. Web download chapter 17 thermochemistry test answer key: Web this section explains how to construct equations and perform calculations that show enthalpy changes for chemical and physical processes.
Energy Stored In Chemical Bonds Of A Substance.
Web start now chapter 17 thermochemistry answers pearson as recognized, adventure as with ease as experience more or less lesson, amusement, as well as promise can be gotten by just checking out a book chapter 17 thermochemistry answers. A process that absorbs heat (^h is positive) exothermic. The energy is transformed into work to move the car. Web add 2+ thermochemical equations to get a final equation, you can add the heats of reaction to get final heat of reaction.
Q Heat Flows From The (Warmer/Cooler) Object To A (Warmer/Cooler) Object Warmer, Cooler What Do Chemical Reactions And Changes In Physical State Generally Involve?
Study of energy changes that occur during chemical reactions and changes in state. How is dynamite used as kinetic energy? The property that is useful for keeping track of heat transfers in chemical. When fuel is burned the products are carbon(soot) carbon monoxide and h2o in.
How Is Gasoline Used As Kinetic Energy?
1 answer in the given process the candle is losing heat as it solidifies this means that the heat is flowing out from. Write the date at the top of the. Chapter 17 thermochemistry test answer key [most popular] 1529 kb/s. This always flows from a.
Web This Section Explains How To Construct Equations And Perform Calculations That Show Enthalpy Changes For Chemical And Physical Processes.
The energy is lost as heat in the exhaust. Metric unit for heat/energy calorie: Chapter 17 thermochemistry test answer key. The energy heats the parts of the engine.