Chapter 3 Chemistry Review
Chapter 3 Chemistry Review - What are the three branches of dalton's theory? A molecular approach by nivaldo tro (the chapter reviews attempt to summarize and explain. Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil. Web general chemistry [1012] chapter 3 review excersise part 1. Nucleus (center of atom, made up of protons and neutrons) 2. University university of massachusetts global; Course chemistry 1 (chem 1010). Click the card to flip 👆. Are made up of two or more substances mixed together. Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium.
Simply, it can be represented as amu. Are the simplest building block of matter. Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil. Web are sipping one while you read.) in this chapter, you will learn to (1) define the terms mixture and compound more precisely, (2) distinguish between elements, compounds, and mixtures, (3) describe how elements. A student collected this information when performing the combustion of a candle experiment: Unit 1 atoms, compounds, and ions. Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium. Web chemistry chapter 3 review questions. What are the three branches of dalton's theory? The value of amu is given by as follows:
Web chemistry library 20 units · 54 skills. Web the following chapter reviews are based on the chapter organization in: Course chemistry 1 (chem 1010). A student collected this information when performing the combustion of a candle experiment: Click the card to flip 👆. Unit 3 more about molecular composition. E = m x c x ∆t. Mass and charge never change 3… Unit 5 chemical reactions and stoichiometry. Web chapter 3.3 review questions and answers term 1 / 24 aps are __________ potentials, that is, they occur full blown or not at all.
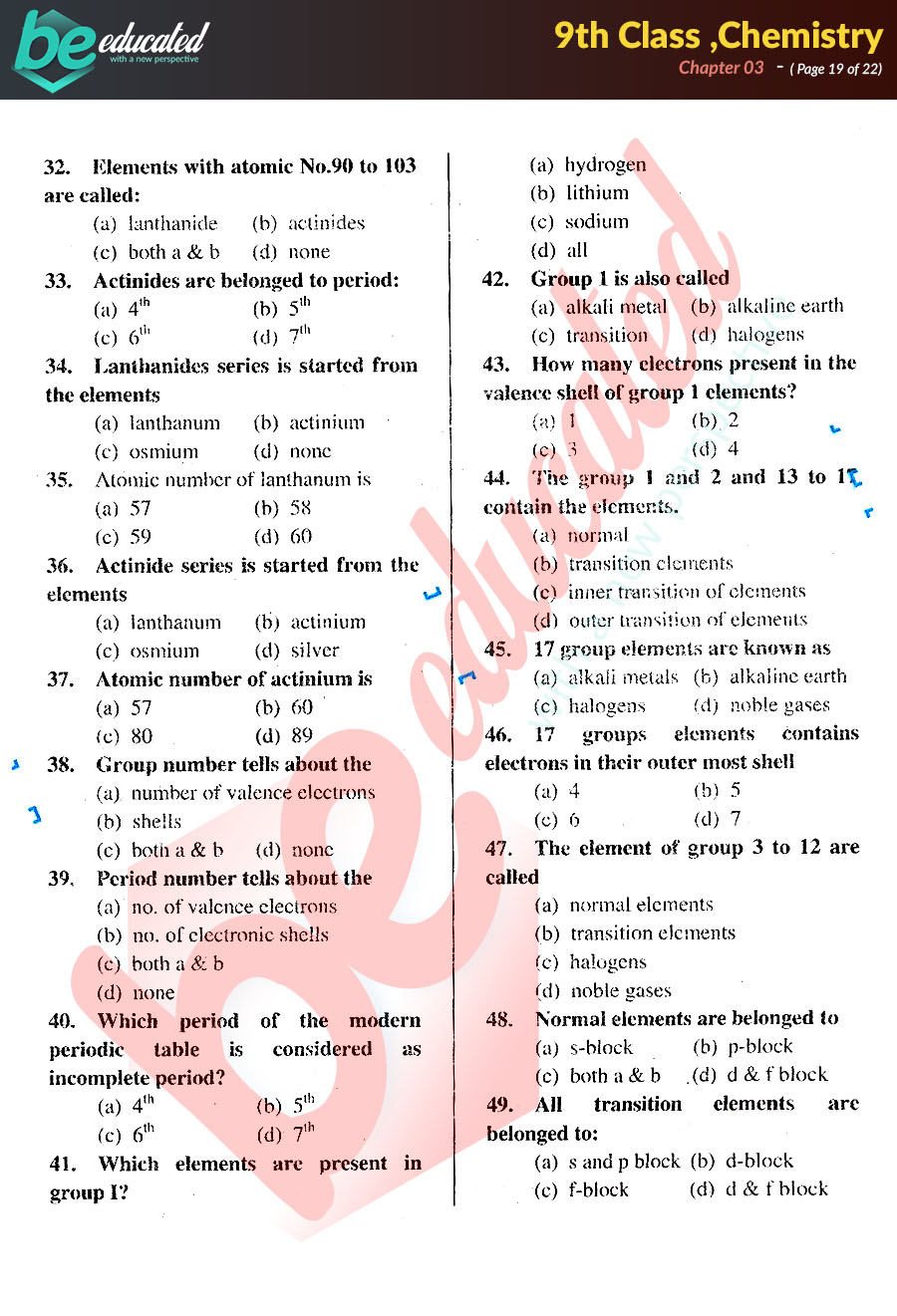
Chapter 3 Chemistry 9th Class Notes Matric Part 1 Notes
Simply, it can be represented as amu. You can find the page numbers for these and many more terms under the key words section at the end of each chapter. Are made up of two or more substances mixed together. Unit 1 atoms, compounds, and ions. Unit 3 more about molecular composition.
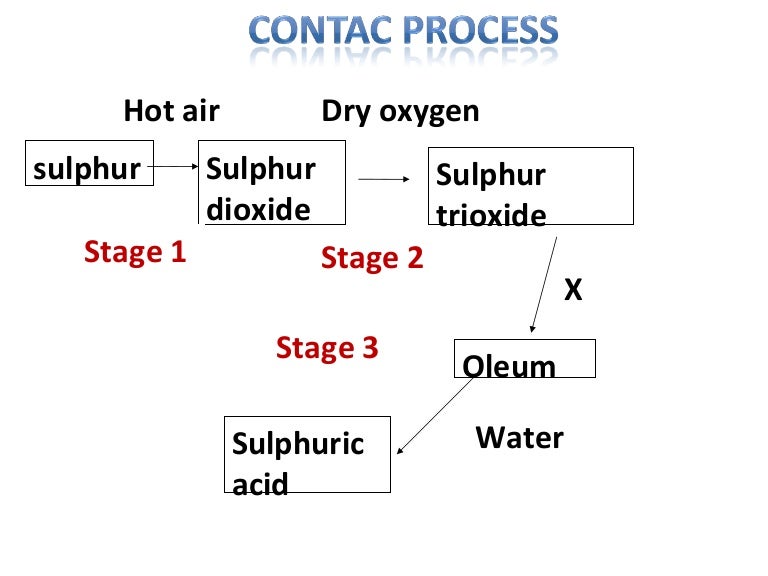
Chemistry Chapter 3
The total mass of the products of a chemical reaction is the same as the total mass of the reactants. Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil. Water temperature = 17 ° c. Simply, it can be represented as amu. Are the simplest building block of matter.
Chemistry chapter 3 number2
Unit 5 chemical reactions and stoichiometry. Web chemistry library 20 units · 54 skills. The value of amu is given by as follows: Web chemistry chapter 3 review questions. University university of massachusetts global;
Class 12th Chemistry Chapter 3 Electrochemistry Complete Study
Web chemistry chapter 3 review questions. Mass and charge never change 3… Law of conservation of mass. Unit 3 more about molecular composition. Web chemistry library 20 units · 54 skills.
Chapter 3 chemistry
Click the card to flip 👆. Present in all atoms and elements 2. Unit 2 more about atoms. Step 2 of 3 (or) step 3 of 3 Web chemistry library 20 units · 54 skills.
Chapter 3 Chemistry 9th Class Notes Matric Part 1 Notes
Web the following chapter reviews are based on the chapter organization in: Web the study of quantitative relationships between chemical formulas and chemical equations. Present in all atoms and elements 2. Unit 3 more about molecular composition. University university of massachusetts global;
Chemistry Form 4 Chapter 3 Spm chemistry form 4 chapter 8 salts
Mass and charge never change 3… Web chemistry chapter 3 review questions. Water temperature = 17 ° c. Law of conservation of mass, law of definite proportions, and law of multiple. Simply, it can be represented as amu.
CHEMISTRY CHAPTER 3 YouTube
A student collected this information when performing the combustion of a candle experiment: Web chemistry library 20 units · 54 skills. Present in all atoms and elements 2. A) heterogeneous mixture b) solution (solid, of copper & zinc) c) solution d) heterogeneous mixture e) solution (gaseous) f) compound g) element h) element i) compound 2. Are the simplest building block.
Chapter 3 Chemistry & Electricity CHI Hair Care Education Portal
University university of massachusetts global; Web chemistry chapter 3 review questions. Law of conservation of mass, law of definite proportions, and law of multiple. Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil. You can find the page numbers for these and many more terms under the key words section at the end of.
Revision on chapter 3 and chapter 9= chemistry
What are the three branches of dalton's theory? It is the standard for measuring the atomic mass of the other elements. Mass and charge never change 3… Web the following chapter reviews are based on the chapter organization in: Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil.
Law Of Conservation Of Mass.
The value of amu is given by as follows: Learn with flashcards, games, and more — for free. Is only one type of atom or molecule (can't be separated) mixture. Nucleus (center of atom, made up of protons and neutrons) 2.
Unit 5 Chemical Reactions And Stoichiometry.
Web are sipping one while you read.) in this chapter, you will learn to (1) define the terms mixture and compound more precisely, (2) distinguish between elements, compounds, and mixtures, (3) describe how elements. Web introduction to chemical bonding. Web chemistry chapter 3 review questions. Simply, it can be represented as amu.
Electrons (Surrounds Nucleus, Made Up Of Negatively Charged Particles) 4 Properties Of Electrons Made By Thomson & Millikan 1.
Present in all atoms and elements 2. Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium. Course chemistry 1 (chem 1010). Law of conservation of mass, law of definite proportions, and law of multiple.
You Can Find The Page Numbers For These And Many More Terms Under The Key Words Section At The End Of Each Chapter.
A molecular approach by nivaldo tro (the chapter reviews attempt to summarize and explain. Web the study of quantitative relationships between chemical formulas and chemical equations. Welcome to my you tube channel geleta abate 1 here’s what you need to know method to score agood results , in physic. A) heterogeneous mixture b) solution (solid, of copper & zinc) c) solution d) heterogeneous mixture e) solution (gaseous) f) compound g) element h) element i) compound 2.