Chapter 7 Chemistry Test
Chapter 7 Chemistry Test - Web chapter 7 chem: Mar 21, 2022 | attempts: Web octet metals are reactive because they lose what easily? Click the card to flip 👆 1 / 11 flashcards learn test. 48 questions | by konlee | updated: Valence electrons what is formed when atoms gain electrons? 7.6 molecular structure and polarity; Web 7.5 strengths of ionic and covalent bonds; N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end?
Negative ions negative ions are called? Web octet metals are reactive because they lose what easily? 7.6 molecular structure and polarity; Mar 21, 2022 | attempts: N2h4(l) + h2(g) → 2nh3(g) reaction 2: Valence electrons what is formed when atoms gain electrons? Click the card to flip 👆 1 / 11 flashcards learn test. Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Web chapter 7 chem: Web 7.5 strengths of ionic and covalent bonds;
Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? 48 questions | by konlee | updated: Click the card to flip 👆 1 / 11 flashcards learn test. Negative ions negative ions are called? Web chapter 7 chem: 7.6 molecular structure and polarity; Web octet metals are reactive because they lose what easily? Mar 21, 2022 | attempts: N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web 7.5 strengths of ionic and covalent bonds;
Chapter 7 Chemistry Test Answers Study Finder
Click the card to flip 👆 1 / 11 flashcards learn test. Web chapter 7 chem: Mar 21, 2022 | attempts: 48 questions | by konlee | updated: N2h4(l) + h2(g) → 2nh3(g) reaction 2:
NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P Block
Mar 21, 2022 | attempts: Web 7.5 strengths of ionic and covalent bonds; 48 questions | by konlee | updated: Click the card to flip 👆 1 / 11 flashcards learn test. N2h4(l) + h2(g) → 2nh3(g) reaction 2:
Chapter 7 Test Review
48 questions | by konlee | updated: Mar 21, 2022 | attempts: N2h4(l) + h2(g) → 2nh3(g) reaction 2: Click the card to flip 👆 1 / 11 flashcards learn test. Web octet metals are reactive because they lose what easily?
modern chemistry chapter 1 test
N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Mar 21, 2022 | attempts: Negative ions negative ions are called? 7.6 molecular structure and polarity;
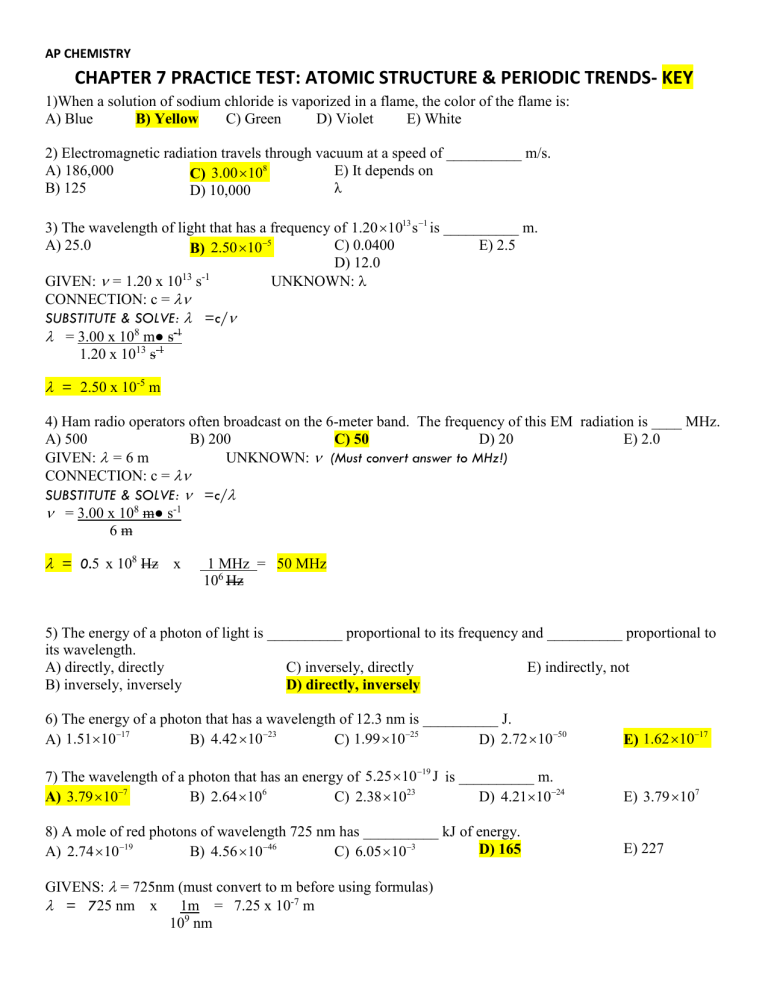
AP CHEMISTRY CHAPTER 7 PRACTICE TEST ATOMIC
Click the card to flip 👆 1 / 11 flashcards learn test. Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Mar 21, 2022 | attempts: N2h4(l) + h2(g) → 2nh3(g) reaction 2: 48 questions | by konlee | updated:
S block elements Chapter 7 Chemistry Class 11 MHTCET 2020 2021
Web 7.5 strengths of ionic and covalent bonds; Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? 48 questions | by konlee | updated: 7.6 molecular structure and polarity; Web octet metals are reactive because they lose what easily?
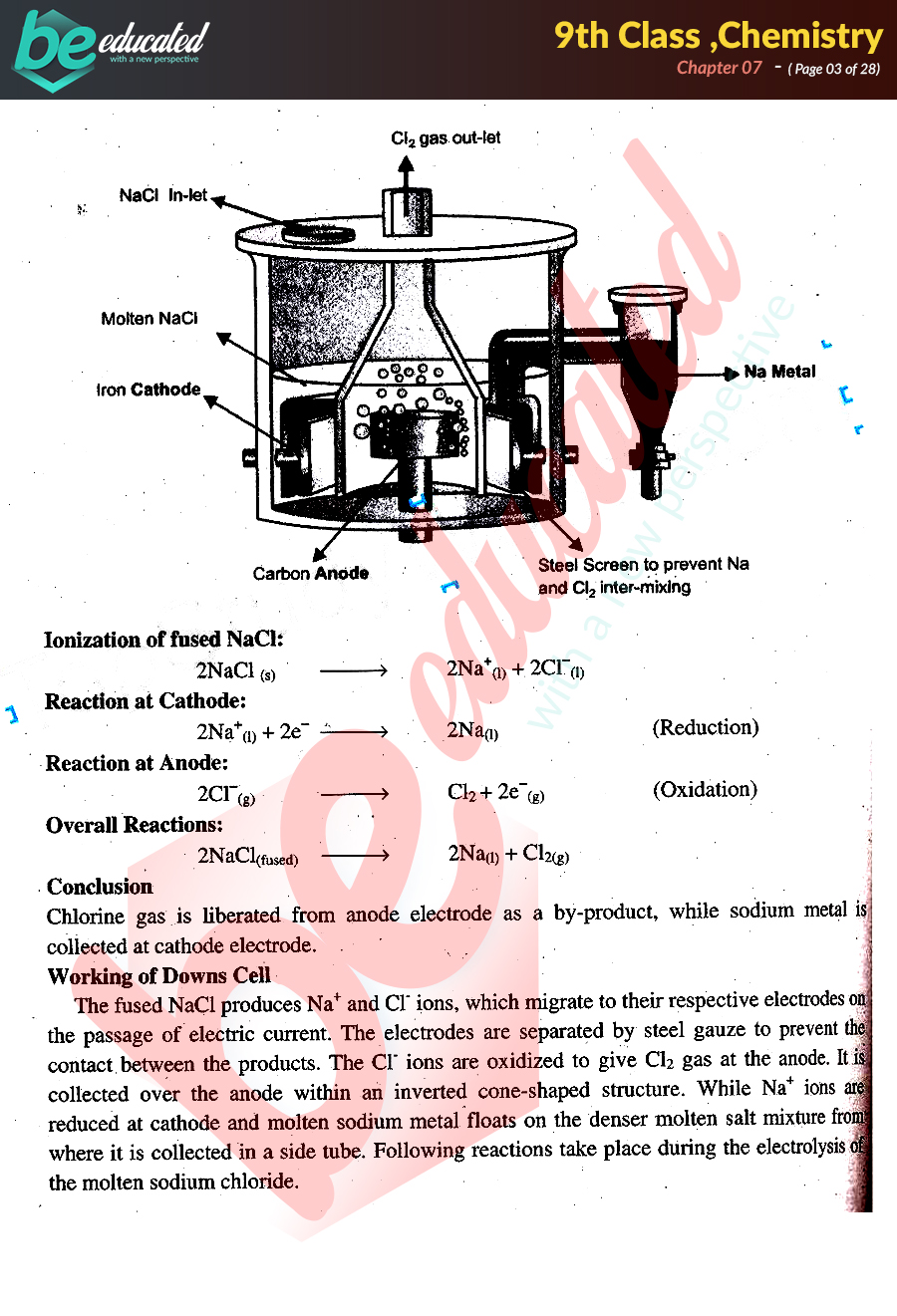
Chapter 7 Chemistry 9th Class Notes Matric Part 1 Notes
Web chapter 7 chem: Valence electrons what is formed when atoms gain electrons? Mar 21, 2022 | attempts: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? 7.6 molecular structure and polarity;
Chapter 7 Chemistry YouTube
Click the card to flip 👆 1 / 11 flashcards learn test. Valence electrons what is formed when atoms gain electrons? Mar 21, 2022 | attempts: 7.6 molecular structure and polarity; Negative ions negative ions are called?
Chemistry Chapter 7 Worksheet Answers —
Negative ions negative ions are called? Click the card to flip 👆 1 / 11 flashcards learn test. Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? 48 questions | by konlee | updated: Valence electrons what is formed when atoms gain electrons?
Negative Ions Negative Ions Are Called?
Mar 21, 2022 | attempts: 48 questions | by konlee | updated: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Web chapter 7 chem:
N2H4(L) + H2(G) → 2Nh3(G) Reaction 2:
Valence electrons what is formed when atoms gain electrons? Web 7.5 strengths of ionic and covalent bonds; 7.6 molecular structure and polarity; Click the card to flip 👆 1 / 11 flashcards learn test.