Chapter 7 Review Chemistry
Chapter 7 Review Chemistry - Energy is defined as the ability to do work. What should a good bonding theory be able to do? Click the card to flip 👆. More about atoms moles and molar mass isotopes unit 3: Only the outer electrons move. Web quick summary of chapter 7: Prelude to stoichiometry this chapter will describe how to symbolize chemical reactions using chemical equations, how to classify some common chemical reactions by identifying patterns of reactivity, and. What is the major conceptual difference between valence bond theory of main group compounds with. Web section 1 short answer c c a. (c) the charge on each fe ion.
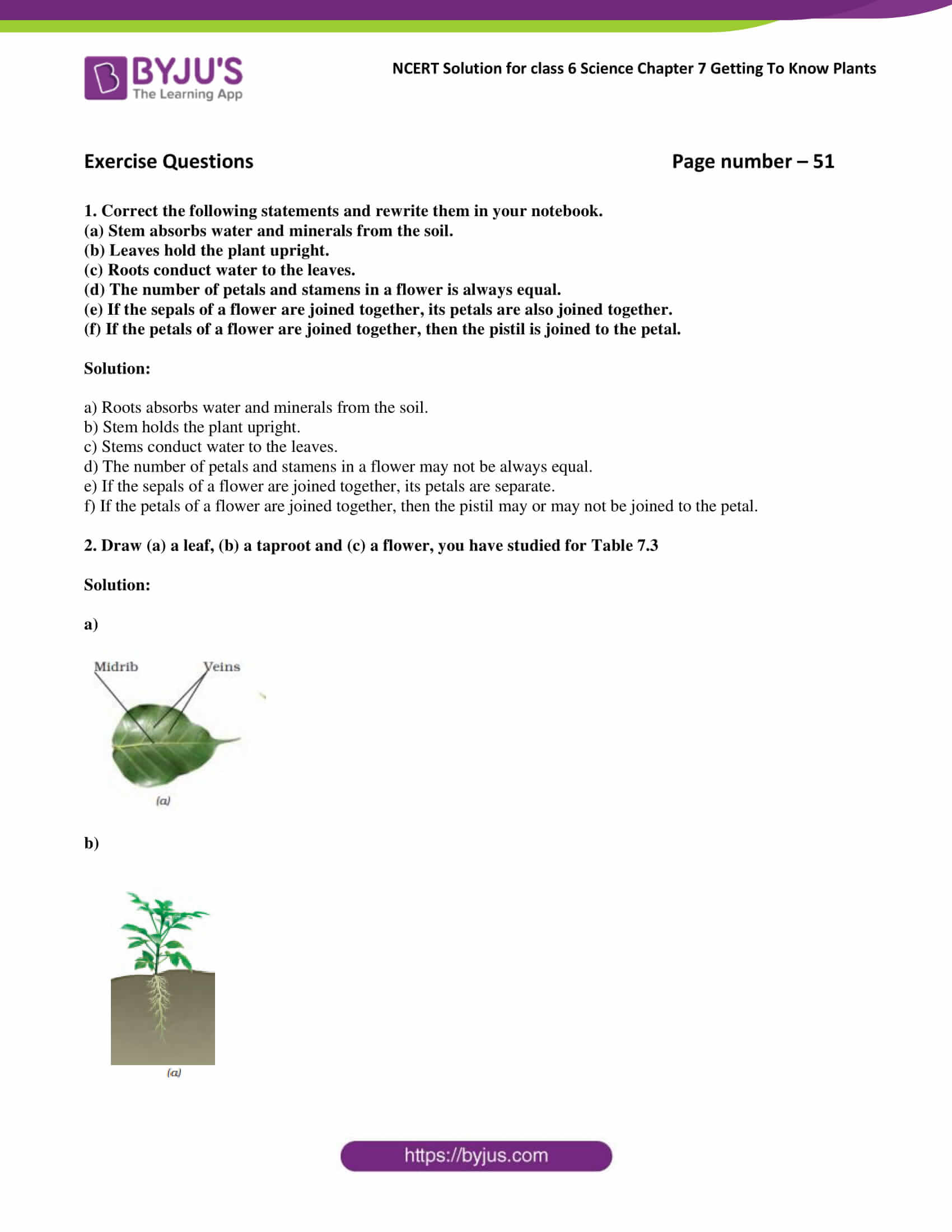
Write formulas for the following compounds: Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed from a single atom (many main group elements). Energy is defined as the ability to do work. Web chapter 7 chemistry review flashcards learn test match chemical formula click the card to flip 👆 shows the kinds and numbers of atoms in the smallest representative unit of a substance click the card to flip 👆 1 / 19 flashcards learn test match created by jackballard vocab chemistry. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. Only the outer electrons move. What should a good bonding theory be able to do? A chemical equation represents a. P, i, cl, and o would form. Web quick summary of chapter 7:
What is the major conceptual difference between valence bond theory of main group compounds with. Number of atoms or ions of each element that are combined in the compound. Web create your own quiz. Only the outer electrons move. Web chemistry chapter 7 review 5.0 (1 review) b. P, i, cl, and o would form. And 7.2 molecular mass, avogadro’s number and the mole what is the total mass (amu and g/mol) of carbon in each of the following molecules? More about atoms moles and molar mass isotopes unit 3: Mar 21, 2022 | attempts: _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit.
PPT Chapter 7 Review PowerPoint Presentation, free download ID2860726
Prelude to stoichiometry this chapter will describe how to symbolize chemical reactions using chemical equations, how to classify some common chemical reactions by identifying patterns of reactivity, and. Write formulas for the following compounds: (c) the charge on each fe ion. Web section 1 short answer c c a. Energy is defined as the ability to do work.
Chemistry, 4th edition Tests Answer Key Christian Liberty
Web create your own quiz. Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions what 3 types of information are used to find an empirical formula from percentage composition. And 7.2 molecular mass, avogadro’s number and the mole what is the total mass (amu and g/mol) of carbon in each of the following molecules?.
5 Best Images of Biochemistry Study Guides And Worksheets Protein
Web chemistry chapter 7 review hno click the card to flip 👆 hyponitrous acid click the card to flip 👆 1 / 19 flashcards learn test match created by clarbour teacher terms in this set (19) hno hyponitrous acid h3po3. Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions what 3 types of information.
Selina Concise Chemistry Class 8 ICSE Solutions Chapter 1 Matter
(c) the charge on each fe ion. Web chemistry chapter 7 review hno click the card to flip 👆 hyponitrous acid click the card to flip 👆 1 / 19 flashcards learn test match created by clarbour teacher terms in this set (19) hno hyponitrous acid h3po3. And 7.2 molecular mass, avogadro’s number and the mole what is the total.
Chapter 7 Test Review 4 YouTube
Click the card to flip 👆. Web chemistry chapter 7 review hno click the card to flip 👆 hyponitrous acid click the card to flip 👆 1 / 19 flashcards learn test match created by clarbour teacher terms in this set (19) hno hyponitrous acid h3po3. Web short answer answer the following questions in the space provided. Mar 21, 2022.
AP CHEMISTRY CHAPTER 7 PRACTICE TEST ATOMIC
Web quick summary of chapter 7: What is the major conceptual difference between valence bond theory of main group compounds with. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. Atoms, compounds, and ions introduction to the atom ions and compounds names and.
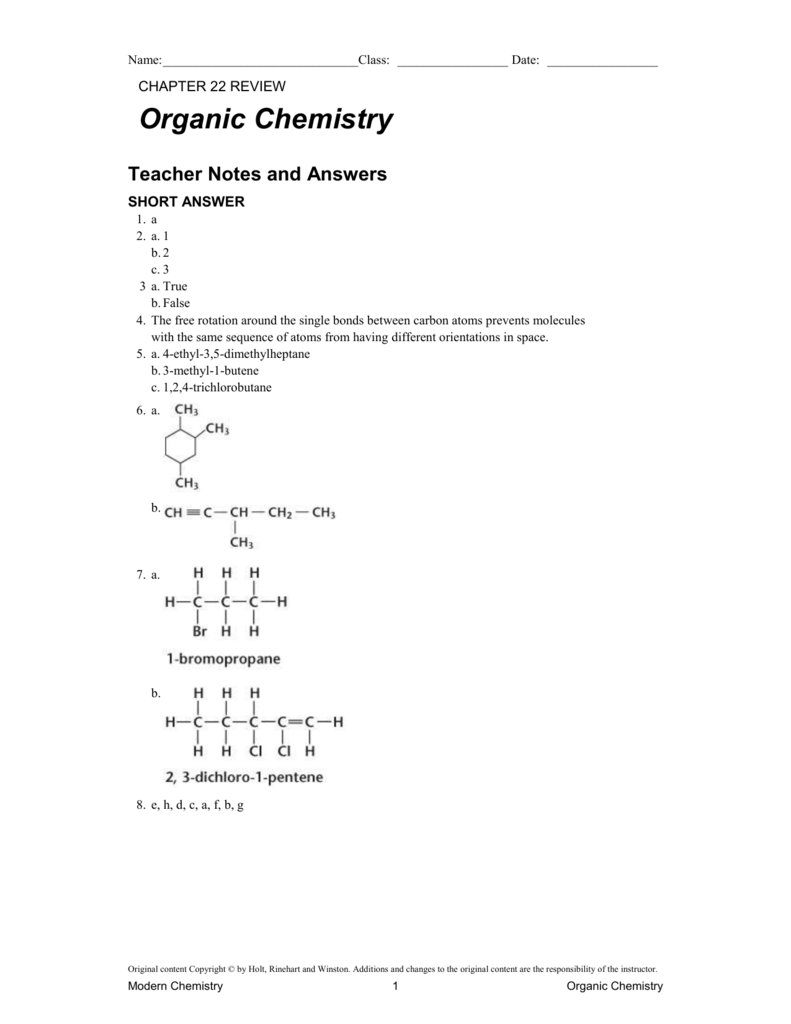
chapter 22 review
4.2 ́ 1024 atoms a. Positive charges form when electrons are lost. And 7.2 molecular mass, avogadro’s number and the mole what is the total mass (amu and g/mol) of carbon in each of the following molecules? _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in.
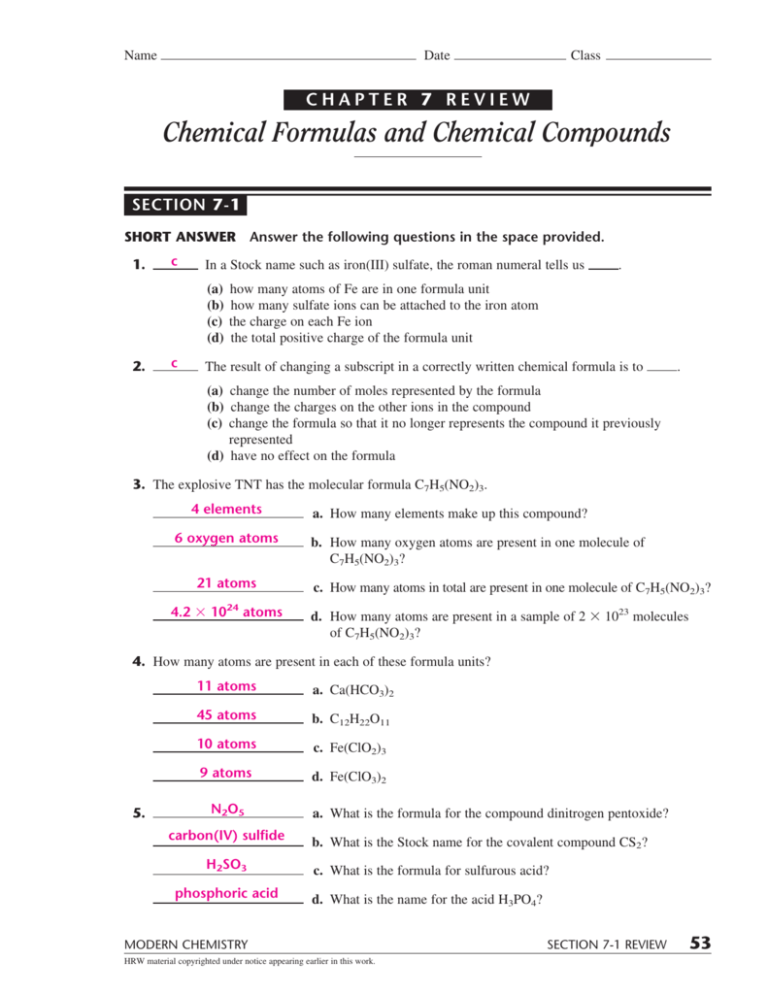
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
_____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. Only the outer electrons move. Web short answer answer the following questions in the space provided. Web created by hlaney04.
Chapter 7 Review
Web chemistry chapter 7 review 5.0 (1 review) b. The block has a constant acceleration and is pulled by means of a horizontal spring that. 18 questions | by muitran | updated: Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed.
Modern Chemistry Chapter 7 Review Answers JackiChuhdary
Prelude to stoichiometry this chapter will describe how to symbolize chemical reactions using chemical equations, how to classify some common chemical reactions by identifying patterns of reactivity, and. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. (d) the total positive charge of.
Web Chemistry Chapter 7 Review 5.0 (1 Review) B.
Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: More about atoms moles and molar mass isotopes unit 3: Only the outer electrons move. Web section 1 short answer c c a.
Click The Card To Flip 👆.
P, i, cl, and o would form. What should a good bonding theory be able to do? (d) the total positive charge of the formula. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit.
(C) The Charge On Each Fe Ion.
What is the major conceptual difference between valence bond theory of main group compounds with. 18 questions | by muitran | updated: Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. A chemical equation represents a.
The Protons In The Nucleus Do Not Change During Normal Chemical Reactions.
Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed from a single atom (many main group elements). Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions what 3 types of information are used to find an empirical formula from percentage composition. The block has a constant acceleration and is pulled by means of a horizontal spring that. Limiting reactant click the card to flip 👆 a.