Chemistry Chapter 3 Test
Chemistry Chapter 3 Test - Web we have compiled the ncert mcq questions for class 11 chemistry chapter 3 classification of elements and periodicity in properties with answers pdf free download covering the entire syllabus. (a) 1.93 × 10 5 c (b) 9.65 × 10 4 c (c) 3.86 × 10 5 c (d) 4.825 × 10 5 c answer question 5. Web learn test match created by cearamaire terms in this set (59) what is an atom? Vocabulary level e unit 6. Find other quizzes for chemistry and more on quizizz for free! Once you complete your mcqs test, you can check the answers and can find your mistake. The study of change 1. Web learn test match created by carlos_dayrit terms in this set (27) a chemical compound always has the same elements in the same proportions by mads regardless of the source of the compound. This is a statement of. Quickly memorize the terms, phrases and much more.
Which one is the most likely reason for an. Diodes click the card to flip 👆 flashcards learn test. Web chapter test a multiple choice in the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. Once you are finished, click the view results button. Find other quizzes for chemistry and more on quizizz for free! Web we have compiled the ncert mcq questions for class 11 chemistry chapter 3 classification of elements and periodicity in properties with answers pdf free download covering the entire syllabus. Element, compound, homogeneous mixture (solution), or heterogeneous mixture: Web answer question 3. Web chapter 3 form 4 quiz for 12th grade students. Rust is a mixture of (a) feo and fe (oh) 3
Transistors click the card to flip 👆 definition 1 / 20 a. Which one is the most likely reason for an. Mr jones chemistry 1 chapter 3. Web learn test match created by cearamaire terms in this set (59) what is an atom? As a result of the strong interelectronic repulsions in fluorine’s. Find other quizzes for chemistry and more on quizizz for free! A box contains some identical red colour balls labelled as a each weighing 2 g. Describe solids, liquids, and gases in terms of density, shape, and volume. Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine. Web learn test match created by nathanb1016 terms in this set (24) pure substance has definite chemical and physical properties homogeneous all of the same or similar kind or nature heterogeneous consisting of.
RHS Honors Chemistry Chemistry Test Answers Chapter 3
Questions will be shuffled each time you start the test. Mr jones chemistry 1 chapter 3. Vocabulary level e unit 6. Web the 9th class chemistry mcqs chapter 3 is available on our website. Describe solids, liquids, and gases in terms of density, shape, and volume.
Chapter 3 Chemistry 9th Class Notes Matric Part 1 Notes
Web chemistry chapter 3 test flashcards learn test match law of multiple proportions click the card to flip 👆 states that when different compounds are formed by a combination of the same elements, different masses of. Determine the molar mass of glucose; Another box contains identical blue colored balls,. Determine the mass of glucose from the number of moles and.
PPT Chemistry Chapter 3 PowerPoint Presentation, free download ID
Any question you have not answered will be marked incorrect. How many coulombs are required for the oxidation of 1 mole of h 2 o to o 2? Web learn test match created by cearamaire terms in this set (59) what is an atom? As a result of the strong interelectronic repulsions in fluorine’s. What is this number expressed in.
chemistry chapter 1 practice test
Web answer question 3. Once you complete your mcqs test, you can check the answers and can find your mistake. Any question you have not answered will be marked incorrect. Web chemistry chapter 3 test flashcards learn test match law of multiple proportions click the card to flip 👆 states that when different compounds are formed by a combination of.
The joy of chemistry a unit in photos High school science, Teaching
Sets found in the same folder. The atomic symbol of silver is —————. Who first suggested the existence of atoms? This is a statement of. Web chemistry chapter 3 quiz for 10th grade students.
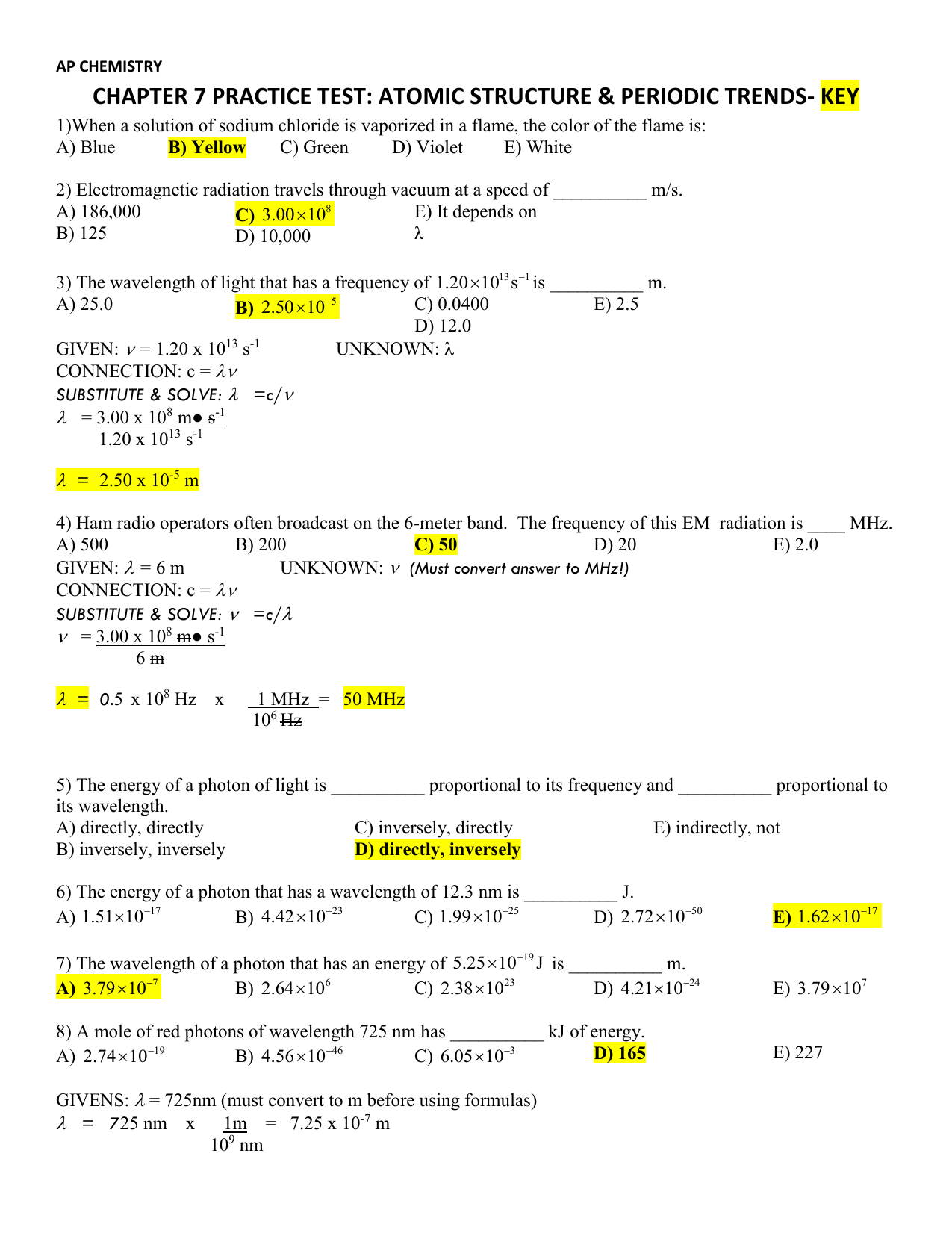
AP CHEMISTRY CHAPTER 7 PRACTICE TEST ATOMIC
Find other quizzes for chemistry and more on quizizz for free! When you complete your online test, the final result. Web answer question 3. (a) 1.93 × 10 5 c (b) 9.65 × 10 4 c (c) 3.86 × 10 5 c (d) 4.825 × 10 5 c answer question 5. Web we have compiled the ncert mcq questions for.
Chapter 5 Practice Problem set 1 answer key 1 Chapter 5 Practice
Mr jones chemistry 1 chapter 3. This is a statement of. Which of the following is a physical. Determine the mass of glucose from the number of moles and its molar mass; Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
modern chemistry chapter 1 test
Once you complete your mcqs test, you can check the answers and can find your mistake. Web ap chemistry test (chapter 3) (november) multiple choice and fib (40%) 1) a chemistry student is filtering and drying a precipitate that formed from two solutions reacting. Questions will be shuffled each time you start the test. Which one is the most likely.
Important questions for class 9 Science Chapter 3 Atoms and Molecules
Web the 9th class chemistry mcqs chapter 3 is available on our website. Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine. This is due to the small size of the fluorine atom. How many significant figures are in the answer to 1.00+9.00. (a) determine the number of moles of glucose in 0.500 l.
Chemistry Played Review Game for Ch 3 Test tomorrow; Study Guide Key
Web chemistry chapter 3 test 3.0 (3 reviews) the diameter of a carbon atom is 0.000 000 000 154 m. Find other quizzes for chemistry and more on quizizz for free! Who first suggested the existence of atoms? Describe solids, liquids, and gases in terms of density, shape, and volume. (a) determine the number of moles of glucose in 0.500.
Another Box Contains Identical Blue Colored Balls,.
This is due to the small size of the fluorine atom. Sets found in the same folder. 9th chemistry chapter 3 online test. Determine the molar mass of glucose;
Which One Is The Most Likely Reason For An.
Which of the following is a physical. What is this number expressed in scientific notation? Web ap chemistry test (chapter 3) (november) multiple choice and fib (40%) 1) a chemistry student is filtering and drying a precipitate that formed from two solutions reacting. Web class 9 atoms and molecules mcqs.
Who First Suggested The Existence Of Atoms?
Mr jones chemistry 1 chapter 3. Transistors click the card to flip 👆 definition 1 / 20 a. (a) 37.0 mol h 2 so 4; Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
Web Answer Question 3.
Web the 9th class chemistry mcqs chapter 3 is available on our website. Once you complete your mcqs test, you can check the answers and can find your mistake. A box contains some identical red colour balls labelled as a each weighing 2 g. Determine the mass of glucose from the number of moles and its molar mass;