Do Metals Form Negative Ions
Do Metals Form Negative Ions - Positively charged ions are called cations, and negatively charged ions are called anions. Web answered • expert verified. Ions can be either monatomic. Most metals become cations when they make ionic compounds. Web metals never form negative ions. Elemental atoms generally lose, gain, or share electrons with other atoms in order to achieve the same electron structure as the. Some atoms have nearly eight electrons in their. Web positively charged ions are called cations. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Metal atoms lose electrons to form positively charged ions.
Most metals become cations when they make ionic compounds. Web metals never form negative ions. Forming an ionic bond, li and f become li + and f − ions. Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web ions form when atoms lose or gain electrons. Web many transition metals can form more than one ion. Chemistry the periodic table metals and nonmetals 1 answer anor277 may 8, 2017 a metal tends to be. What is unique about the electron configurations of. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Negative ions, by gaining electrons to fill the valence shell negative ions, by losing.
Positively charged ions are called cations, and negatively charged ions are called anions. Most metals become cations when they make ionic compounds. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. What type of ions do metals naturally form? Web the name of a metal ion is the same as the name of the metal atom from which it forms,. Some atoms have nearly eight electrons in their. Web metals never form negative ions. Web ions form when atoms lose or gain electrons to obtain a full outer shell: What is unique about the electron configurations of. Thus, the electron affinity will be.
Electron Affinity of The Elements
Web no, metals do not form negative ions: Thus, the electron affinity will be. Web thus, nonmetals tend to form negative ions. Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. To obtain a full outer shell:
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
Web no, metals do not form negative ions: Metal atoms lose electrons to form positively charged ions. Web positively charged ions are called cations. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web the name of a metal ion is the same as the name of.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web answered • expert verified. What type of ions do metals naturally form? Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Transition metals have a wide variety of applications. To obtain a full outer shell:
Chem matters ch6_ionic_bond
Web metals never form negative ions. Web no, metals do not form negative ions: Ions can be either monatomic. Elemental atoms generally lose, gain, or share electrons with other atoms in order to achieve the same electron structure as the. Forming an ionic bond, li and f become li + and f − ions.
Formation of Negative Ions SPM Chemistry
What type of ions do metals naturally form? Web positively charged ions are called cations. Metal atoms lose electrons to form positively charged ions. Web no, metals do not form negative ions: Negative ions, by gaining electrons to fill the valence shell negative ions, by losing.
Chem matters ch6_ionic_bond
Forming an ionic bond, li and f become li + and f − ions. Ions can be either monatomic. Web the name of a metal ion is the same as the name of the metal atom from which it forms,. What is unique about the electron configurations of. Web positively charged ions are called cations.
Periodic Table With Charges Of Ions Elcho Table
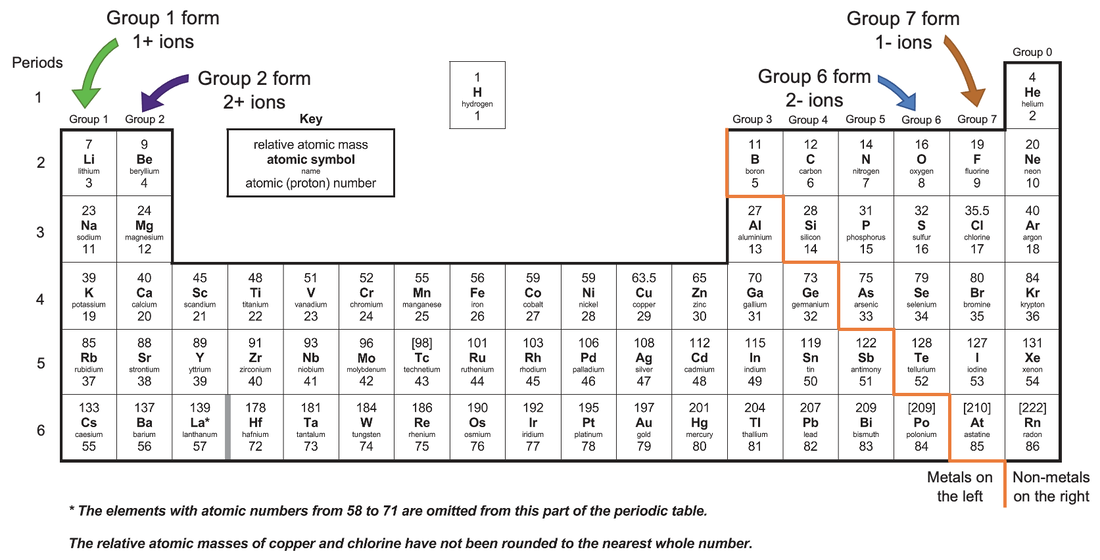
Web many transition metals can form more than one ion. Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an.
Chem matters ch6_ionic_bond
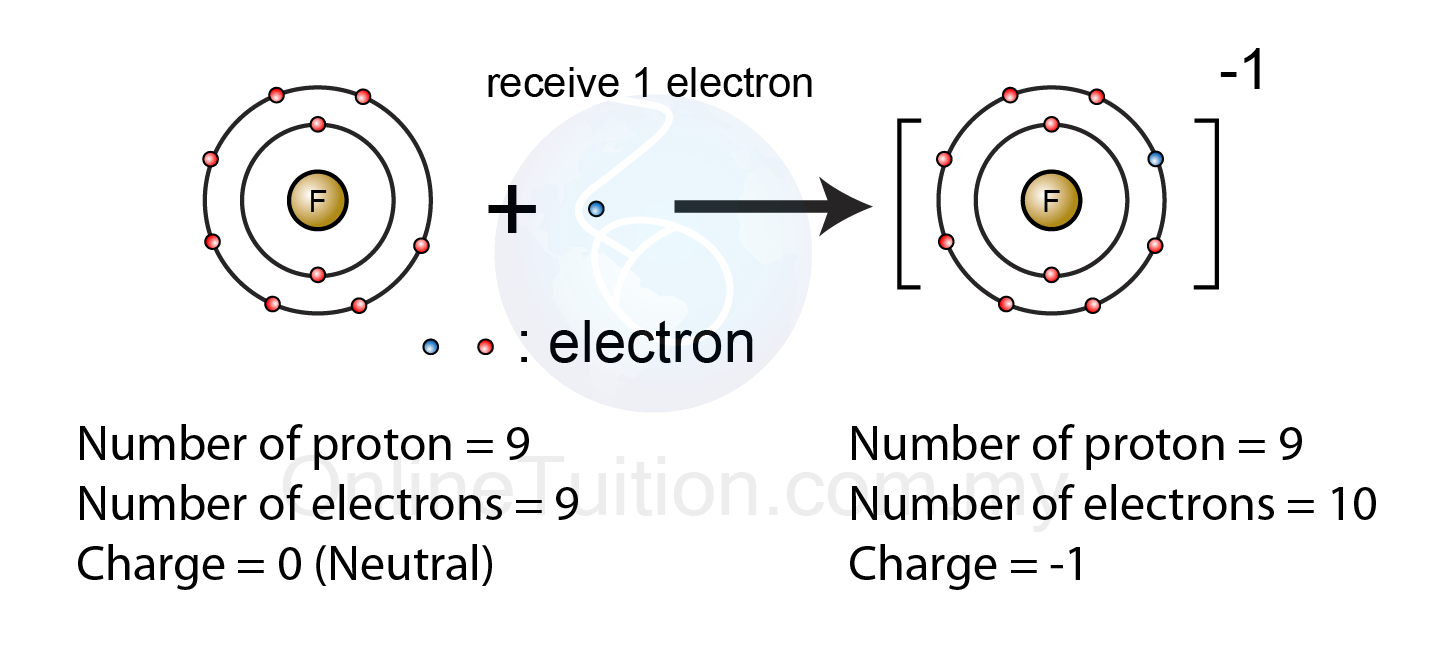
Positively charged ions are called cations, and negatively charged ions are called anions. Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. To obtain a full outer shell: Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Electron transfer between lithium (li).
Chem matters ch6_ionic_bond
What is unique about the electron configurations of. Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web many transition metals can form more than one ion. Chemistry the periodic table metals and nonmetals 1 answer anor277 may.
Do metals form anions or cations quizlet? Book Vea
To obtain a full outer shell: Forming an ionic bond, li and f become li + and f − ions. Most metals become cations when they make ionic compounds. Web ions form when atoms lose or gain electrons. Web the name of a metal ion is the same as the name of the metal atom from which it forms,.
Electron Transfer Between Lithium (Li) And Fluorine (F).
Transition metals have a wide variety of applications. Elemental atoms generally lose, gain, or share electrons with other atoms in order to achieve the same electron structure as the. Ions can be either monatomic. Web answered • expert verified.
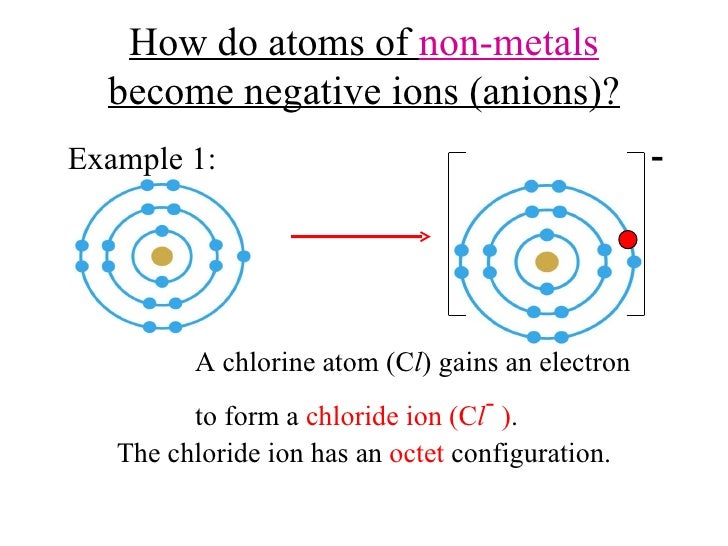
Web 1 2 3 4 5 6 7 Forming Negative And Positive Ions Forming Negative Ions (Anions) Atoms Gain Electrons In Their Outer Shell When They Form Negative Ions, Called Anions.
Positively charged ions are called cations, and negatively charged ions are called anions. Web no, metals do not form negative ions: What type of ions do metals naturally form? Some atoms have nearly eight electrons in their.
Web Metals Never Form Negative Ions.
Web many transition metals can form more than one ion. Metal atoms lose electrons to form positively charged ions. Web ions form when atoms lose or gain electrons. Chemistry the periodic table metals and nonmetals 1 answer anor277 may 8, 2017 a metal tends to be.
Negative Ions, By Gaining Electrons To Fill The Valence Shell Negative Ions, By Losing.
Most metals become cations when they make ionic compounds. Thus, the electron affinity will be. Web thus, nonmetals tend to form negative ions. To obtain a full outer shell: