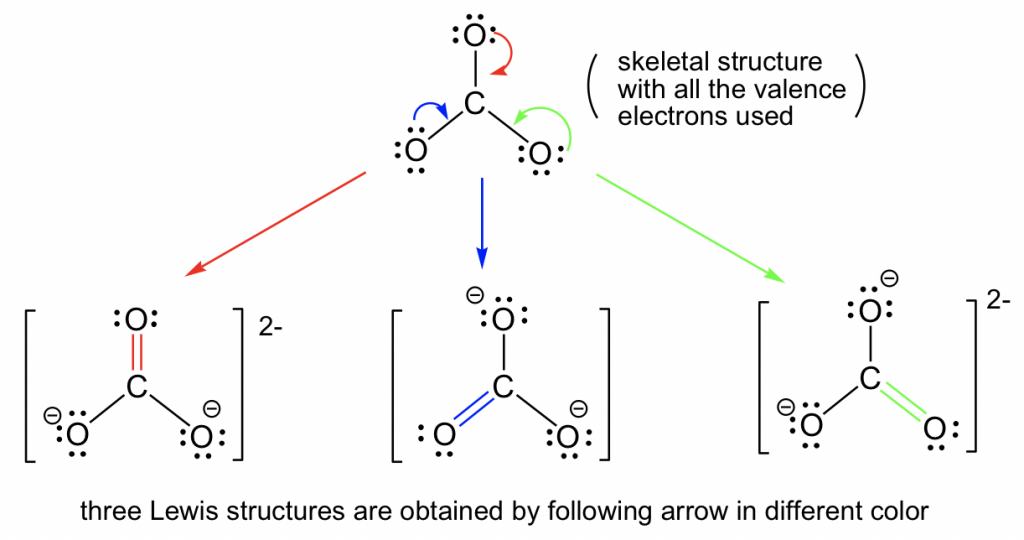
Draw A Lewis Structure For Co32
Draw A Lewis Structure For Co32 - In carbonate ion, among the two elements, carbon has an. Obeys the octet rule b. Web the following lewis diagram represents the valence electron configuration. Lewis structure of carbonate ion is drawn in this tutorial step by step. Write lewis structures that obey the octet rule for each of the following molecules. The negative charge on the ion is located on two of the oxygen atoms, to indicate. Here’s the best way to solve it. (valence electrons are the number of electrons present in the. Web draw the lewis structure of the carbonate ion, co 32−. Web see the big list of lewis structures.
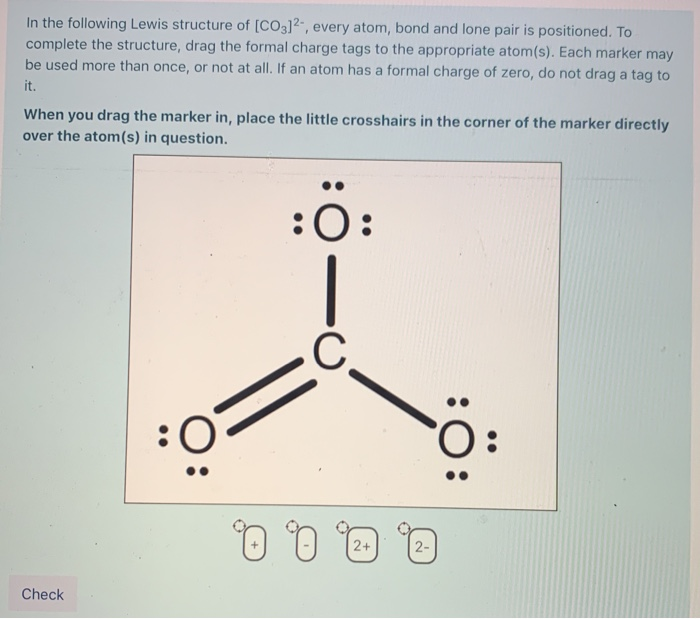
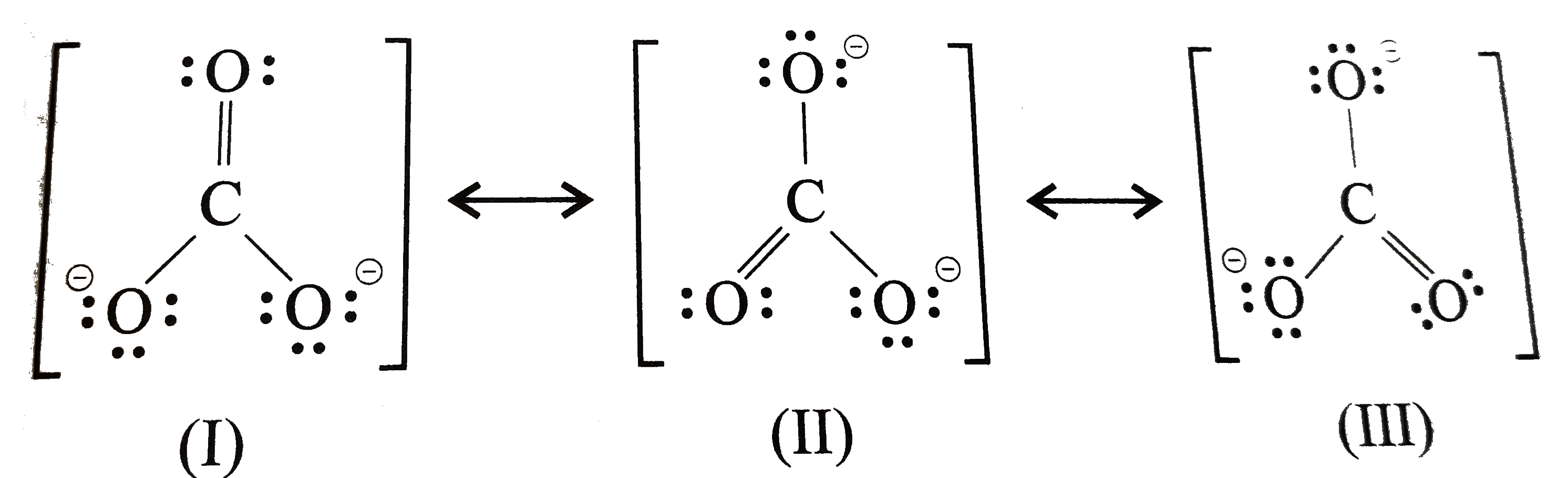
I also go over the resonance, hybridization, shape and bond angle. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. Start by counting the total number of valence electrons in the molecule/ion. For the central carbon atom: Determine the central atom of the molecule. The central carbon atom a. In this case, carbon (c) contributes 4 valence electrons, and each oxygen (o) contributes 6 valence electrons, giving a total of 24 valence. Which of the following statements is true? In carbonate ion, among the two elements, carbon has an. Describe one resonance structure of the carbonate ion.
= 4 + 6*3 + 2. Draw the lewis structure for co32, including any valid resonance structures. For the central carbon atom: In carbonate ion, among the two elements, carbon has an. You will learn about these facts in this tutorial. The remaining electrons are distributed as lone pairs on the oxygen atoms. Start by counting the total number of valence electrons in the molecule/ion. In the lewis structure, the outermost orbit electrons of each atom is shown. Here’s the best way to solve it. The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐.
1.3 Resonance Structures Chemistry LibreTexts
The lewis structure for co2− 3 is shown in the figure. Web here’s the best way to solve it. Start by counting the total number of valence electrons in the molecule/ion. 4 plus 18 plus 2: Lewis structure of carbonate ion is drawn in this tutorial step by step.
Draw the Lewis structure for CO3^2
Oxygen has six, we have 3 oxygens, and this negative 2 means we have an extra two valence electrons. Write lewis structures that obey the octet rule for each of the following molecules. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Web draw the lewis structure of the carbonate ion, co.
Solved In the following Lewis structure of [CO3)2, every
Carbon has 4 valence electrons; Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Web see the big list of lewis structures. (valence electrons are the number of electrons present in the. Icl in each case, the atom listed first is the central atom.
CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
Six electrons are used, and 6 are left over. Icl in each case, the atom listed first is the central atom. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. This video discusses the resonance structu. Carbon is the least electronegative, put that at.
Draw the Lewis Structure for Co32
Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Carbon is the least electronegative, put that at. I also go over the resonance, hybridization, shape and bond angle. Write lewis structures that obey the octet rule for each of the following molecules. Web draw lewis structures for the following species.
CO32 Lewis Structure, Characteristics 13 Facts You Should Know
The lewis structure for co2− 3 is shown in the figure. Carbon is the least electronegative, put that at. The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. In this.
CO32 Molecular Geometry, Shape and Bond Angles (Carbonate Ion) YouTube
I also go over the resonance, hybridization, shape and bond angle. This can be calculated by multiplying the valence electrons of each atom. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Carbon is located in group 14 of the periodic table and has four valence electrons, while oxygen, belonging to group 16, has six.
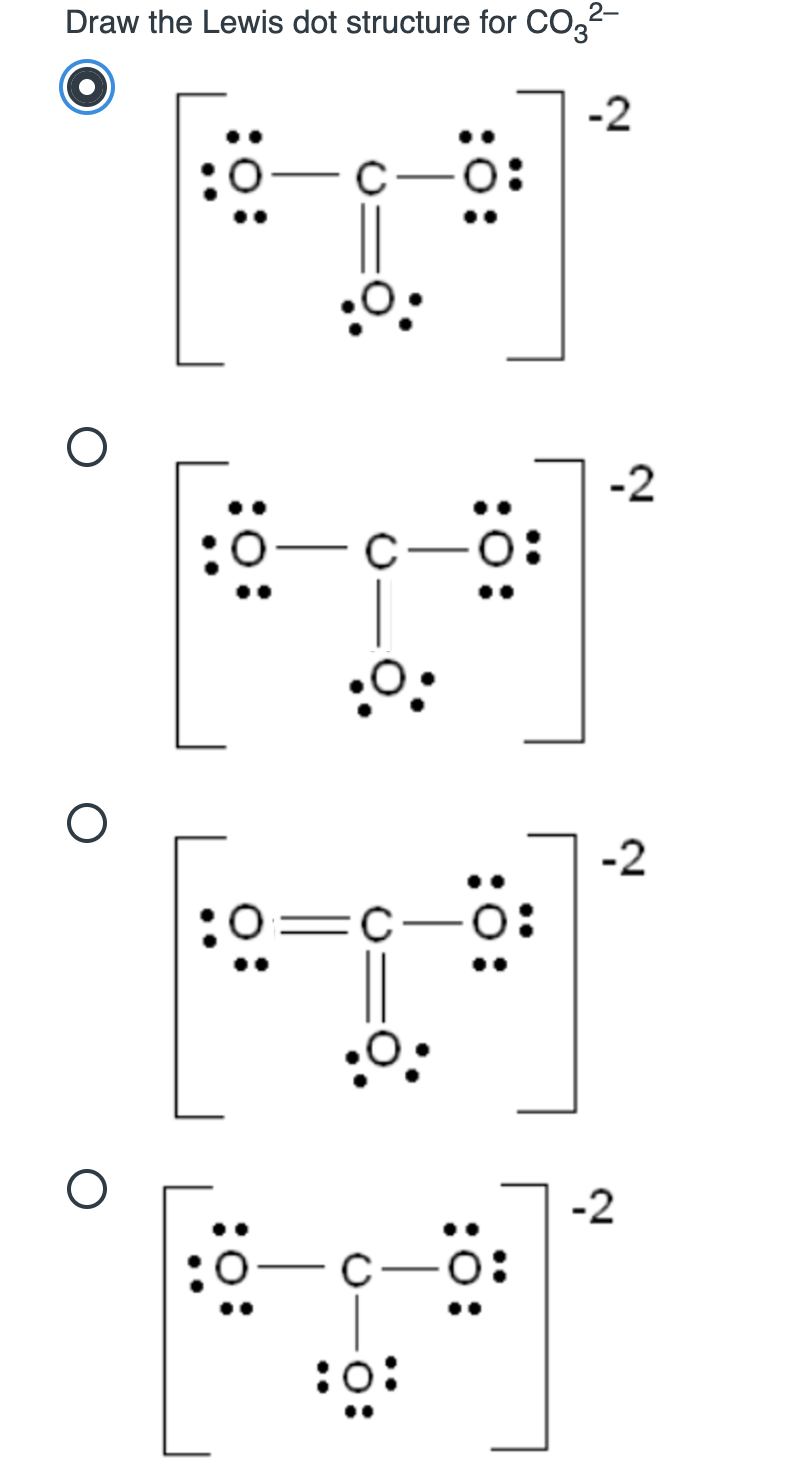
Solved Draw the Lewis dot structure for CO32 2 0 O 2 0
Web see the big list of lewis structures. Here’s the best way to solve it. Here’s the best way to solve it. The central carbon atom a. Which of the following statements is true?, give the number of valence.
Explain the structure of CO(3)^(2) ion in terms of resonance
Which of the following statements is true? 4 plus 18 plus 2: Draw the lewis structure for co32, including any valid resonance structures. Here’s the best way to solve it. Which of the following statements is true?, give the number of valence.
How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube
This can be calculated by multiplying the valence electrons of each atom. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. Start by counting the total number of valence electrons.
The Remaining Electrons Are Distributed As Lone Pairs On The Oxygen Atoms.
I also go over the resonance, hybridization, shape and bond angle. Start by counting the total number of valence electrons in the molecule/ion. Single bonds and 1c =o double. Now, in order to draw the lewis structure, we have to determine which one is the central atom in a multiatomic heterogeneous molecule, here an ion.
You Will Learn About These Facts In This Tutorial.
Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. Icl in each case, the atom listed first is the central atom. (valence electrons are the number of electrons present in the. Describe one resonance structure of the carbonate ion.
Draw The Lewis Structure For Co32, Including Any Valid Resonance Structures.
Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral. Here’s the best way to solve it. Which of the following statements is true?, give the number of valence. The lewis structure for co2− 3 is shown in the figure.
Web Draw Lewis Structures For The Following Species.
Carbon is located in group 14 of the periodic table and has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. (the skeleton is indicated by the way the molecule is written.) (a) cl2co (b) h3c—cn (c) h2c—ch2. The number of unshared pairs (lone pairs) on the central catom is: The negative charge on the ion is located on two of the oxygen atoms, to indicate.