Draw A Six Carbon Alkyne That Can Exist As Diastereomers
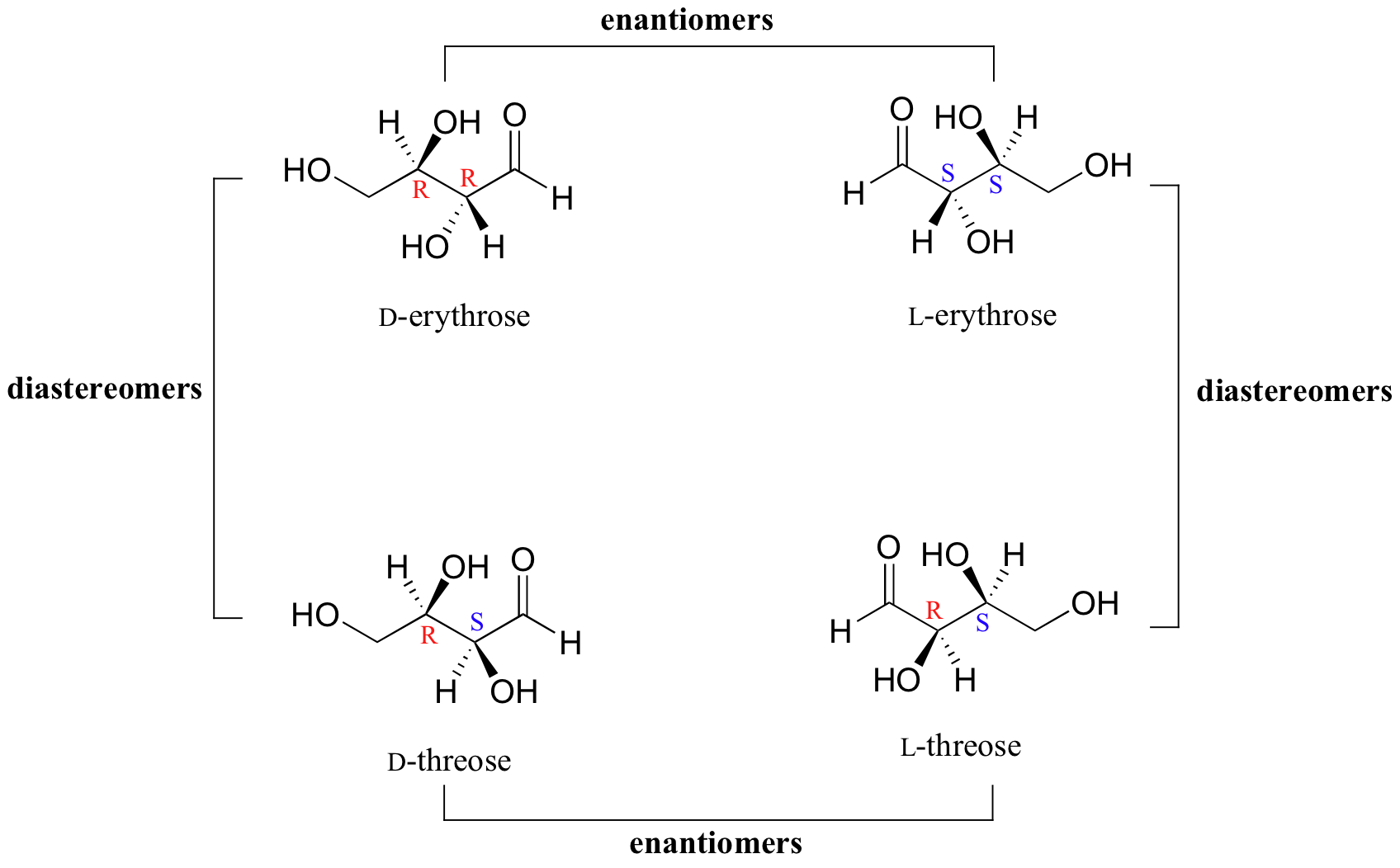
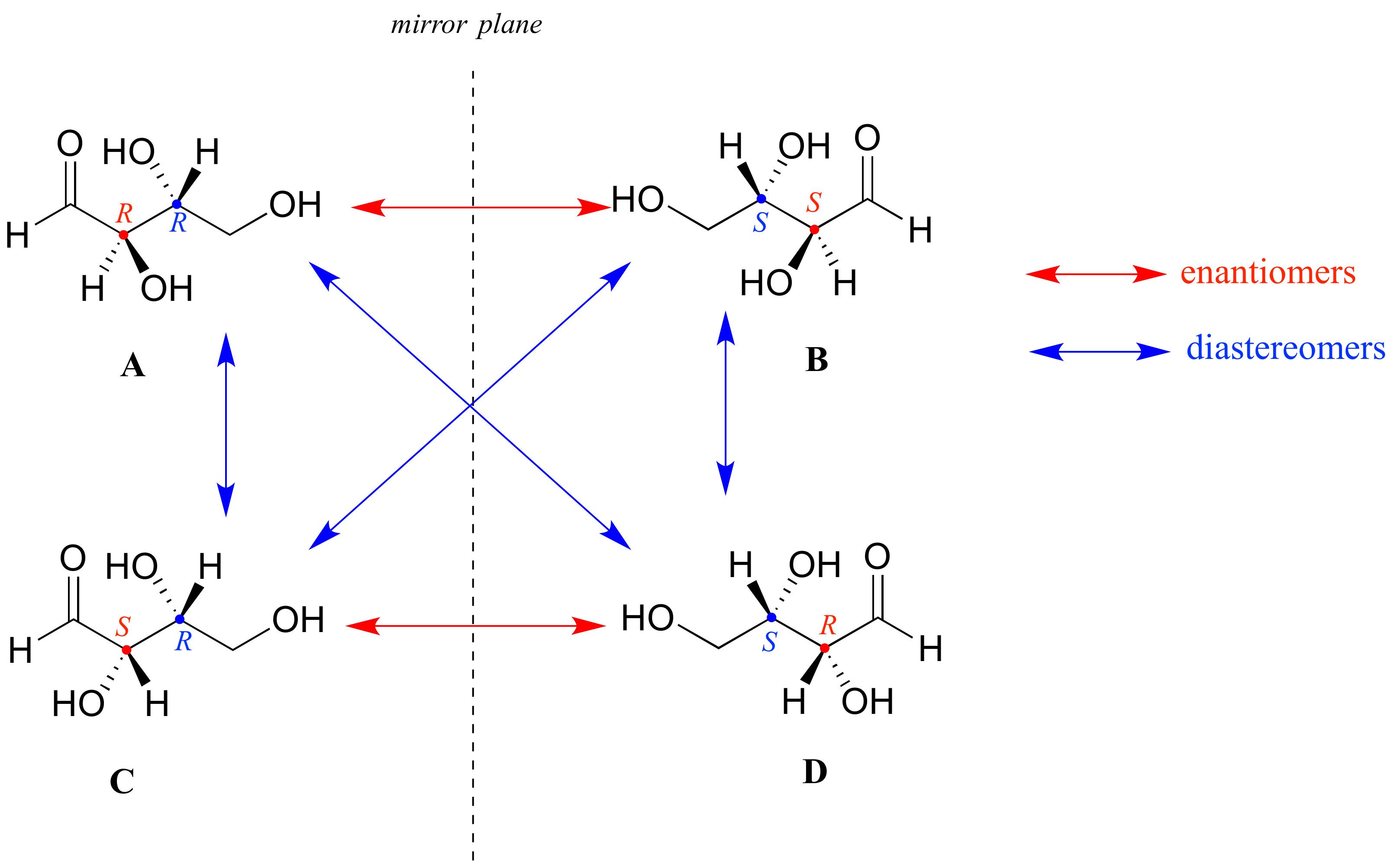
Draw A Six Carbon Alkyne That Can Exist As Diastereomers - Web because they are not mirror images, they must be diastereomers. You do not need to specify wedge or dashed bonds. Web as a general rule, a molecule with n chirality centers can have up to 2 n stereoisomers (although it may have fewer, as we’ll see below). Since threonine has two chirality centers (c2 and c3), there are four possible stereoisomers, as shown in figure 5.11. Talk to an expert about this answer. Draw a six carbon alkyne that can exist as diastereomers. (the molecule should contain only carbon and hydrogen atoms.) here’s the best way to solve it. One way for a compound to have diastereomers is to have two chiral centers. This is why jay talks about pairs of diastereomer*s* or enantiomer*s*. This alkyne does not have any stereocenters, so it cannot exist as diastereomers.
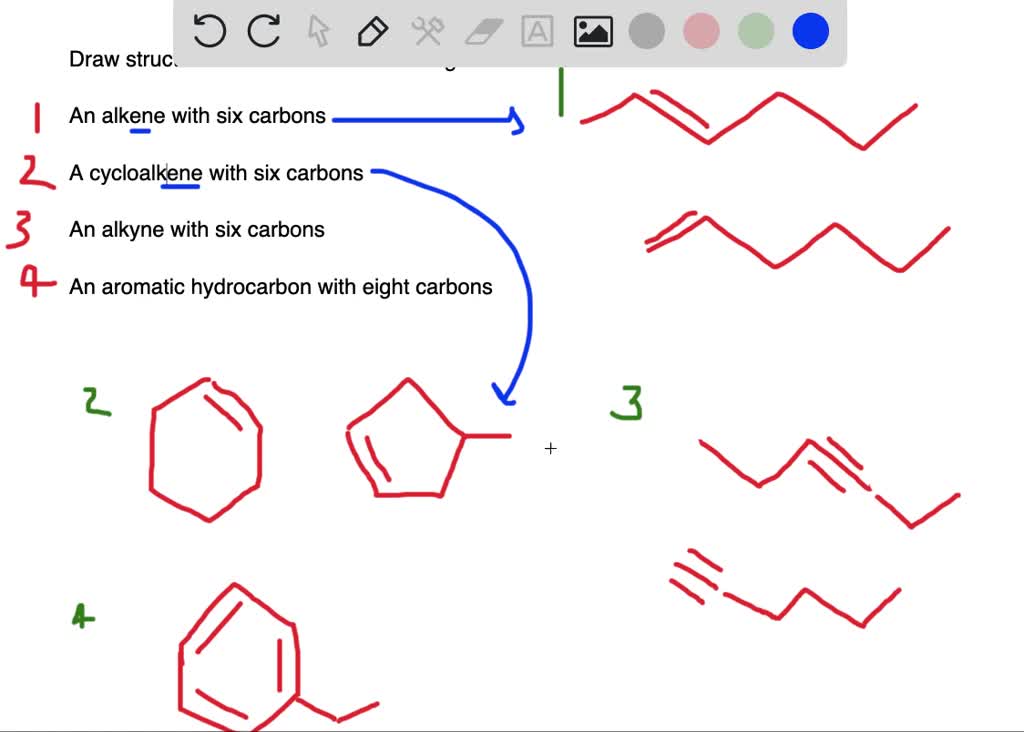
An al kind is a molecule with a triple bond. This alkyne does not have any stereocenters, so it cannot exist as diastereomers. 100% (18 ratings) share share. (the molecule should contain only carbon and hydrogen atoms.) organic compounds: I will add a triple bond and at least… You do not need to specify wedge or dashed bonds. 5.2 the reason for handedness in molecules: Molecule should contain only carbon and hydrogen atoms.) please answer this question its due thanks. This problem has been solved! The molecule should only contain only carbon and hydrogen atoms.i really need help with this problem.
Web because they are not mirror images, they must be diastereomers. Molecule should contain only carbon and hydrogen atoms.) please answer this question its due thanks. You do not need to specify wedge or dashed bonds. Thus, in most cases a diastereomer is also an. You do not need to specify wedge or dashed bonds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 9.6 oxidative cleavage of alkynes; 5.6 • diastereomers molecules like lactic acid, alanine, and glyceraldehyde are relatively simple because each has only one chirality center and thus. 5.2 the reason for handedness in molecules: An al kind is a molecule with a triple bond.
Diastereomers Chemistry LibreTexts
An alkene group which can exist in two stereoisomeric forms is referred to as stereogenic. (the molecule should contain only carbon and hydrogen atoms.) organic compounds: Be sure to indicate stereochemistry and use hyphens (−) not endashes (−). 5.6 • diastereomers molecules like lactic acid, alanine, and glyceraldehyde are relatively simple because each has only one chirality center and thus..
SOLVEDDraw at least two structural formulas for each of the following
Iverson, eric anslyn, christopher s. Thus, in most cases a diastereomer is also an. You do not need to specify wedge or dashed bonds. Since threonine has two chirality centers (c2 and c3), there are four possible stereoisomers, as shown in figure 5.11. We can introduce the concept of.
Answered Draw a sixcarbon alkyne that can exist… bartleby
Web an individual molecule is an enantiomer or a diastereomer of another molecule — i.e. This alkyne does not have any stereocenters, so it cannot exist as diastereomers. Talk to an expert about this answer. You do not need to specify wedge or dashed bonds. (the molecule should contain only carbon and hydrogen atoms.) here’s the best way to solve.
Solved Draw A Sixcarbon Alkyne That Can Exist As Diaster...
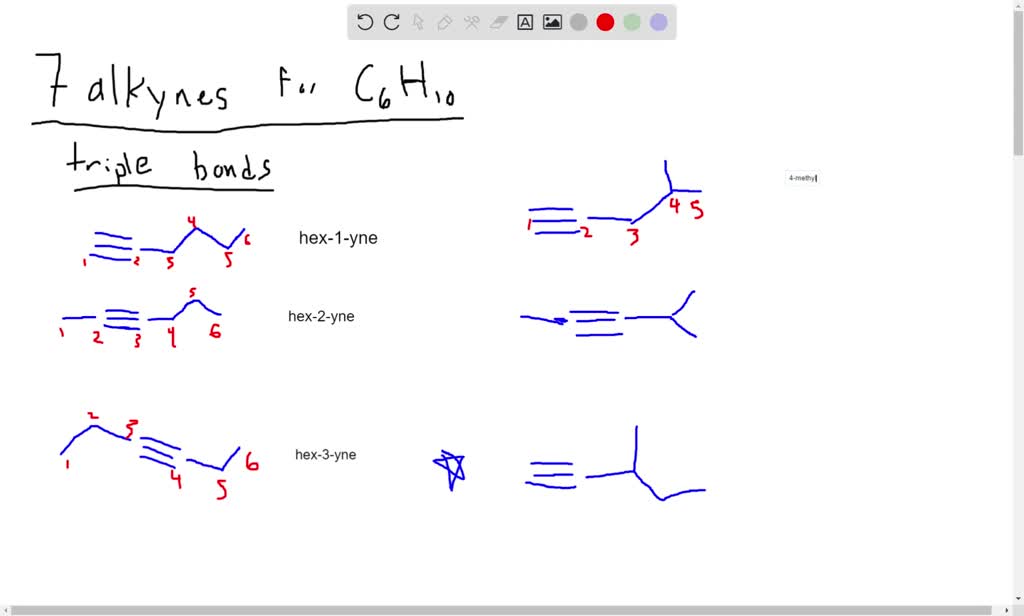
The molecule should only contain only carbon and hydrogen atoms.i really need help with this problem. It has a triple bond between the third and fourth carbon atoms, with the remaining atoms forming a chain. 9.6 oxidative cleavage of alkynes; One way for a compound to have diastereomers is to have two chiral centers. Thus, in most cases a diastereomer.
5.6 Diastereomers Chemistry LibreTexts
H h h h h h. Web an individual molecule is an enantiomer or a diastereomer of another molecule — i.e. 5.2 the reason for handedness in molecules: (the molecule should contain only carbon and hydrogen atoms.) organic chemistry. This problem has been solved!
6.4 Diastereomers more than one chiral center Chemistry LibreTexts
One way for a compound to have diastereomers is to have two chiral centers. Select draw rings more erase / mich 5 3 2 o. An alkene group which can exist in two stereoisomeric forms is referred to as stereogenic. This alkyne does not have any stereocenters, so it cannot exist as diastereomers. You'll get a detailed solution from a.
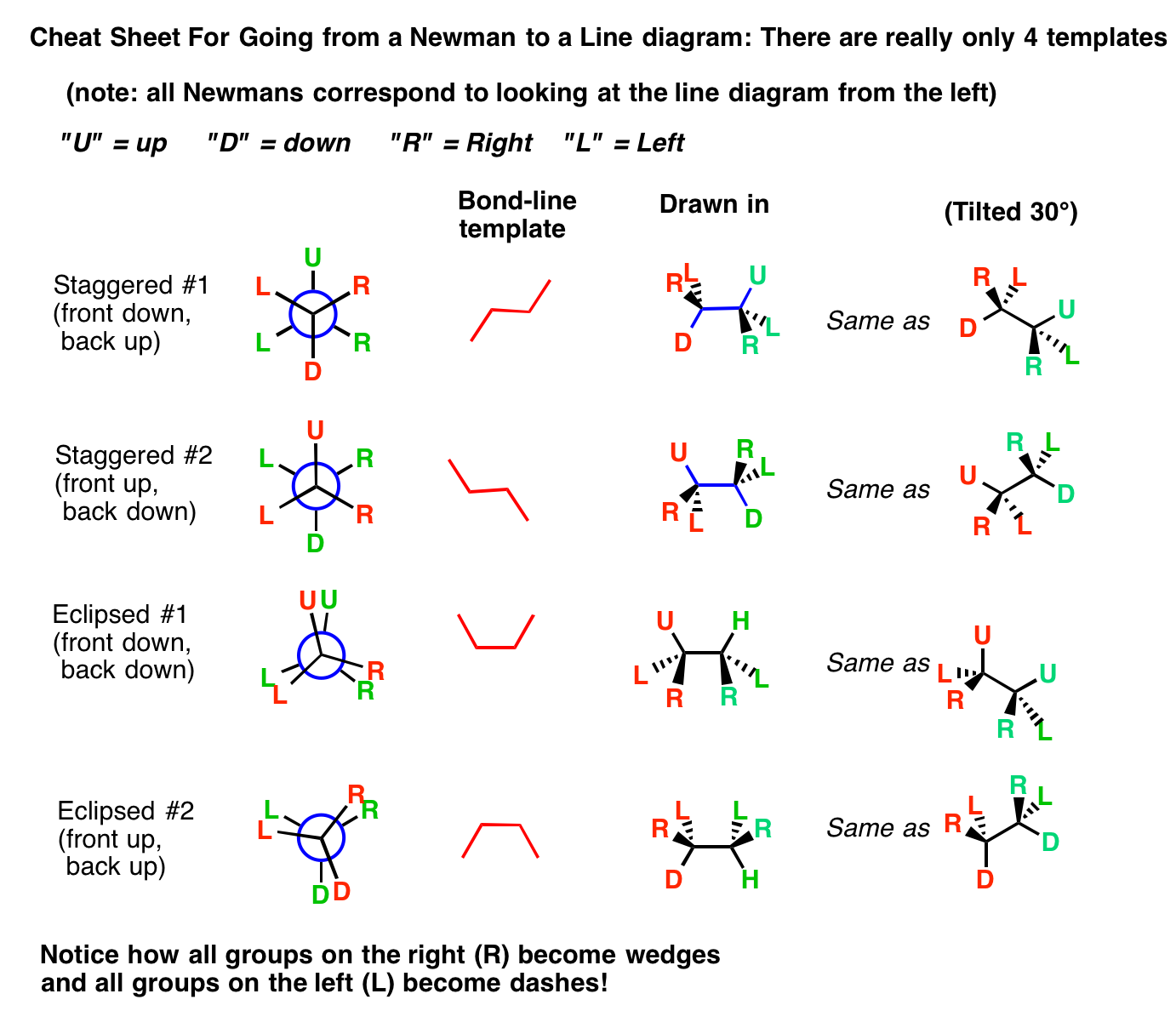
How would you draw Newman projections of alkanes? Socratic
5.2 the reason for handedness in molecules: Web an individual molecule is an enantiomer or a diastereomer of another molecule — i.e. You do not need to specify wedge or dashed bonds. Web 5.1 enantiomers and the tetrahedral carbon; An alkene group which can exist in two stereoisomeric forms is referred to as stereogenic.
SOLVEDDraw the structures and give the common and systematic names for
Web 5.1 enantiomers and the tetrahedral carbon; Iverson, eric anslyn, christopher s. 5.2 the reason for handedness in molecules: The molecule should only contain only carbon and hydrogen atoms.i really need help with this problem. You do not need to specify wedge or dashed bonds.
2.1 Structures of Alkenes Chemistry LibreTexts
Draw a six carbon alkyne that can exist as diastereomers. Molecule should contain only carbon and hydrogen atoms.) please answer this question its due thanks. 5.2 the reason for handedness in molecules: We can introduce the concept of. One way for a compound to have diastereomers is to have two chiral centers.
Draw A Six Carbon Alkyne That Can Exist As Diastereomers Drawing
(the molecule should contain only carbon and hydrogen atoms.) organic compounds: An alkene group which can exist in two stereoisomeric forms is referred to as stereogenic. An al kind is a molecule with a triple bond. Talk to an expert about this answer. Web 5.1 enantiomers and the tetrahedral carbon;
5.2 The Reason For Handedness In Molecules:
This is why jay talks about pairs of diastereomer*s* or enantiomer*s*. It has a triple bond between the third and fourth carbon atoms, with the remaining atoms forming a chain. Web an individual molecule is an enantiomer or a diastereomer of another molecule — i.e. 5.6 • diastereomers molecules like lactic acid, alanine, and glyceraldehyde are relatively simple because each has only one chirality center and thus.
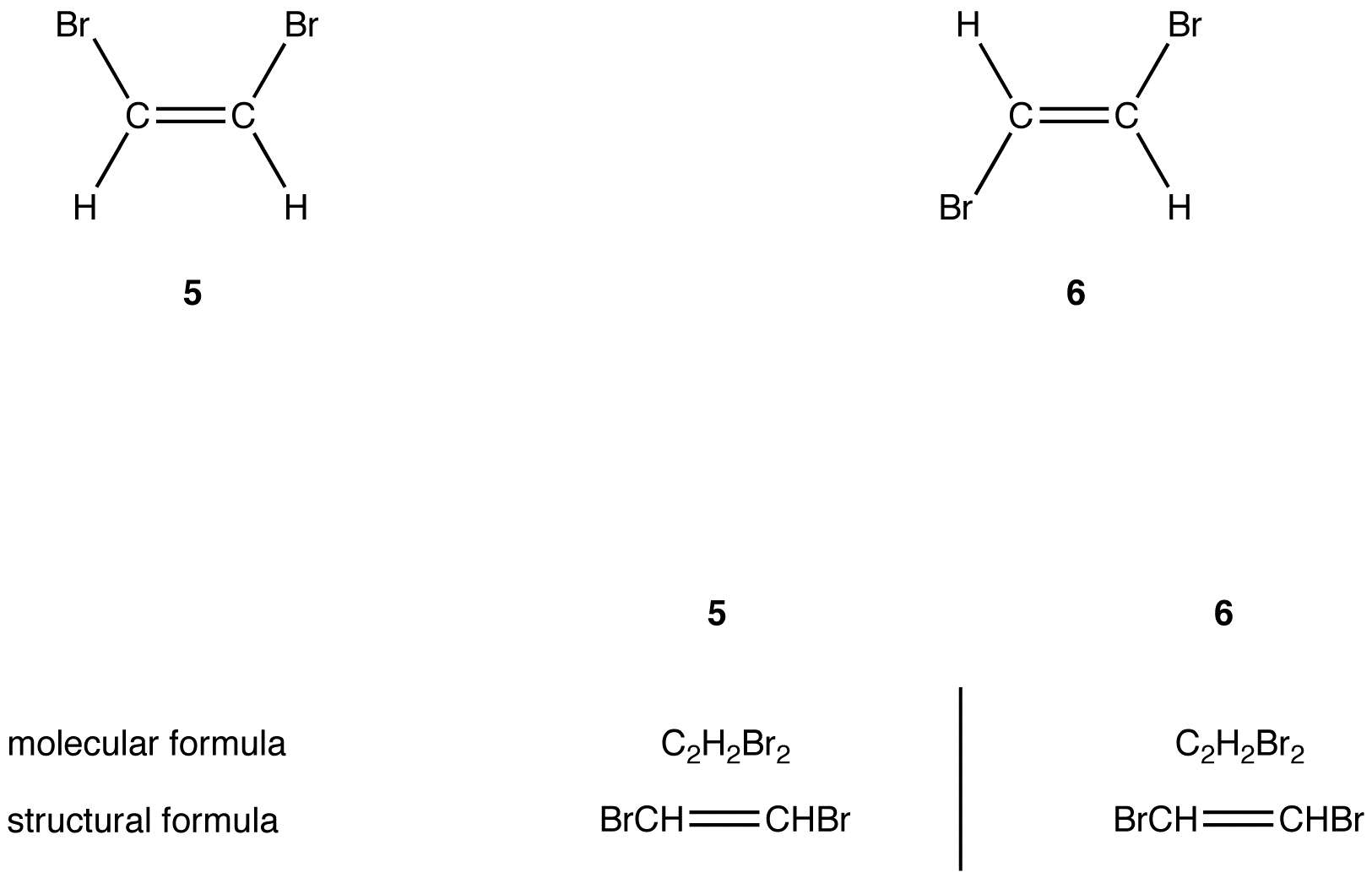
Web Because They Are Not Mirror Images, They Must Be Diastereomers.
Draw a six carbon alkyne that can exist as diastereomers. Talk to an expert about this answer. An alkene group which can exist in two stereoisomeric forms is referred to as stereogenic. You do not need to specify wedge or dashed bonds.
Be Sure To Indicate Stereochemistry And Use Hyphens (−) Not Endashes (−).
Web because they are not mirror images, they must be diastereomers. Web 5.1 enantiomers and the tetrahedral carbon; You do not need to specify wedge or dashed bonds. We can introduce the concept of.
You Do Not Need To Specify Wedge Or Dashed Bonds.
This problem has been solved! This alkyne does not have any stereocenters, so it cannot exist as diastereomers. (the molecule should contain only carbon and hydrogen atoms.) organic compounds: To know more about diastereomers visit: