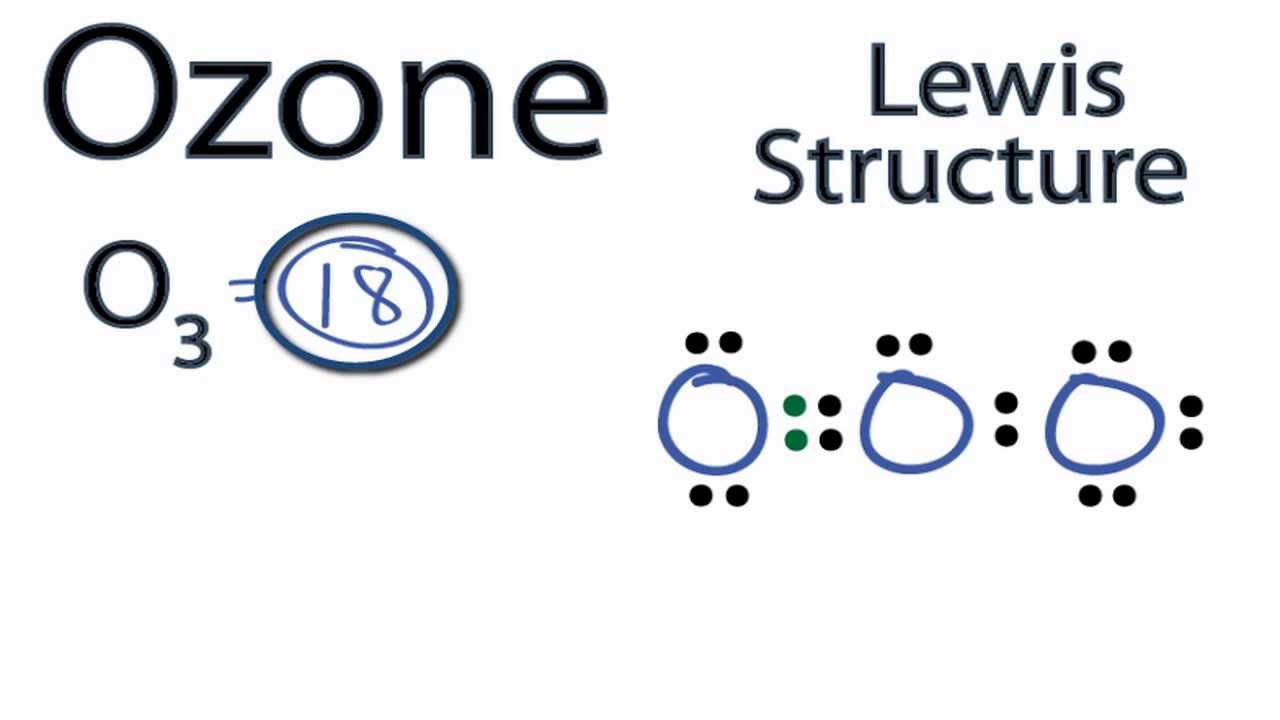
Draw All Resonance Structures For The Ozone Molecule O3
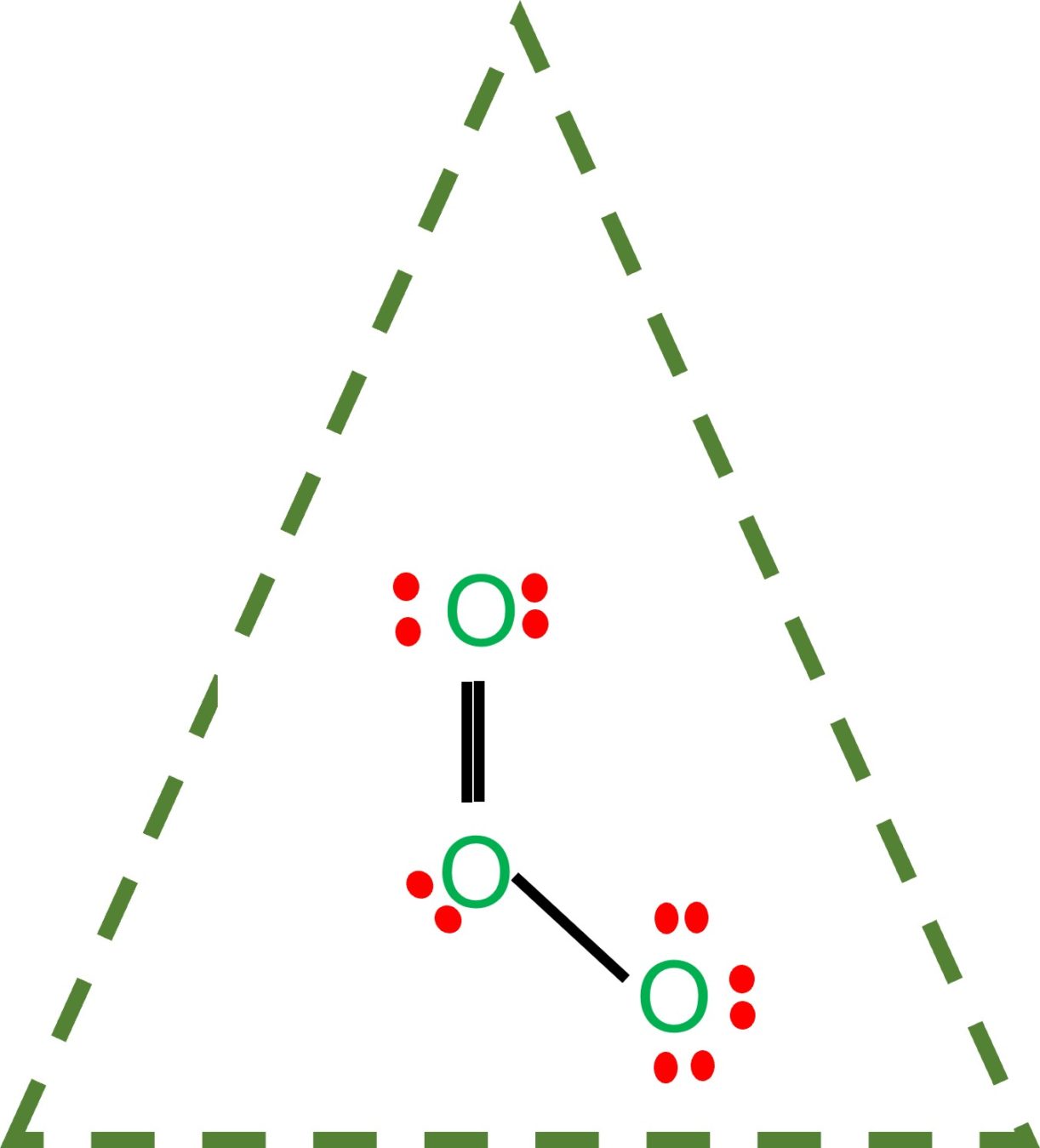
Draw All Resonance Structures For The Ozone Molecule O3 - Ozone is a trigonal planar molecule. Web here is a diagrammatic representation of the mo diagram of ozone. We're using all 18 valence electrons. Ozone, o 3, an unstable, blue, diamagnetic gas with a characteristic pungent odor, protects the earth. Web the first resonance structure shown below has one positively charged oxygen, one negatively charged oxygen and one neutral oxygen with two bonds. Web some molecules or ions cannot be adequately described by a single lewis structure. The total number of valence. (i) resonating structure of ozone ( o 3 ): I quickly take you through how to draw the lewis structure of o3 (ozone). In this post, we will be drawing the lewis structure of ozone, o 3.
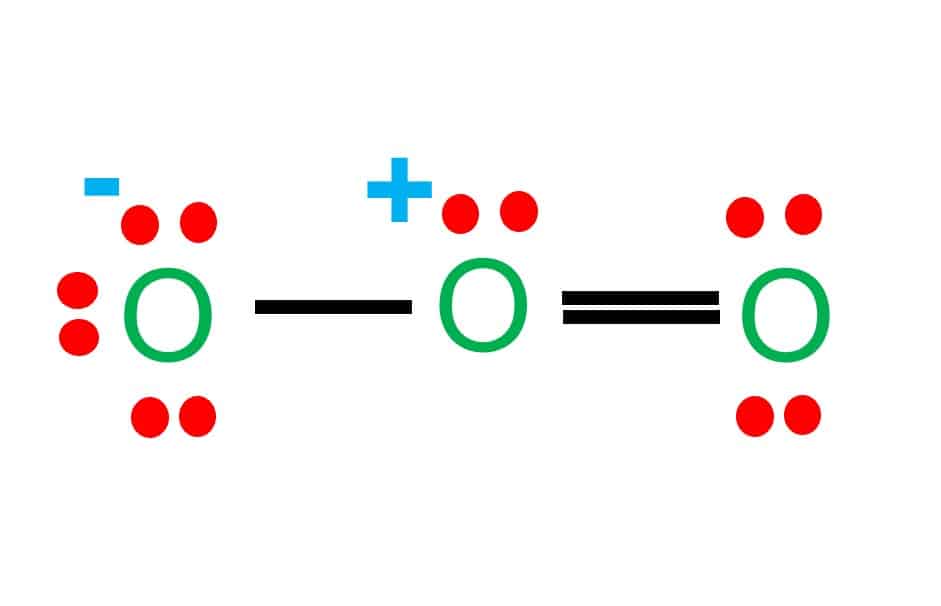
Web draw all resonance structures for the ozone molecule, o 3. Web if there are equivalent resonance structures, draw all of them. Web the first resonance structure shown below has one positively charged oxygen, one negatively charged oxygen and one neutral oxygen with two bonds. (i) resonating structure of ozone ( o 3 ): Hence, as we take one p orbital from each atom of oxygen. In this post, we will be drawing the lewis structure of ozone, o 3. Web this lesson walks you through each step of drawing the lewis structure of o 3. (figure 2) was this answer helpful? Ozone, o 3, an unstable, blue, diamagnetic gas with a characteristic pungent odor, protects the earth. Web resonance structures of o3.
After drawing the lewis structure of nh 3, the shape of the o 3 molecule can be. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Ozone, o 3, an unstable, blue, diamagnetic gas with a characteristic pungent odor, protects the earth. This chemistry video tutorial explains how to draw the. Web draw all resonance structures for the ozone molecule, o 3. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). In ozone, a molecular orbital extending over all three oxygen. We're using all 18 valence electrons. Hence, as we take one p orbital from each atom of oxygen. I quickly take you through how to draw the lewis structure of o3 (ozone).
O3 Lewis Structure Step By Step Drawing What's Insight
Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Web ozone, or #o_3#, has two major resonance structures that contribute equally to the overall hybrid structure of the molecule. Ozone is a trigonal planar molecule. Web the first resonance structure shown below has one positively charged oxygen,.
O3 Resonance Structures (Ozone) YouTube
(i) resonating structure of ozone ( o 3 ): The total number of valence. 85k views 3 years ago new ap & general chemistry video playlist. Web if there are equivalent resonance structures, draw all of them. Web resonance structures of o3.
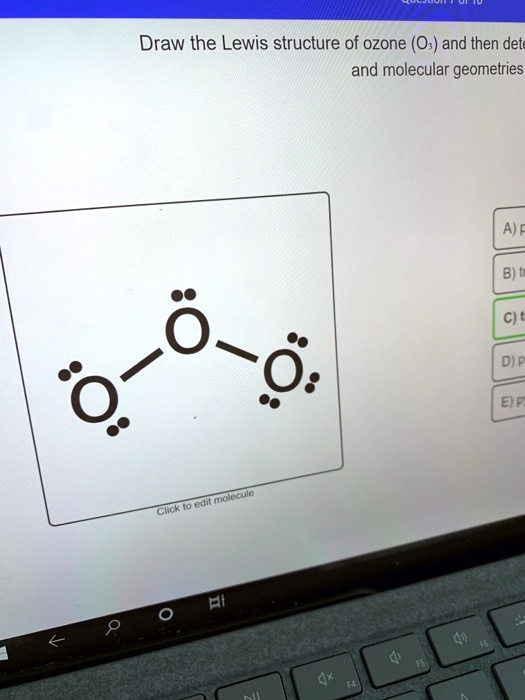
SOLVED Draw the Lewis structure of ozone (O3) and then determine its
The total number of valence. This chemistry video tutorial explains how to draw the. We can draw o3 different ways. In this post, we will be drawing the lewis structure of ozone, o 3. (figure 2) was this answer helpful?
Ozone Molecule Lewis Structure
Ozone, o 3, an unstable, blue, diamagnetic gas with a characteristic pungent odor, protects the earth. Web if there are equivalent resonance structures, draw all of them. Web resonance structures of o3. Web draw all resonance structures for the ozone molecule, o 3. (figure 1) (ii) resonating structure of n o 3 −:
Resonance Presentation Chemistry
Web figure 5.3.4 the resonance structure of ozone involves a molecular orbital extending all three oxygen atoms. Web ozone, or #o_3#, has two major resonance structures that contribute equally to the overall hybrid structure of the molecule. We can draw o3 different ways. (figure 1) (ii) resonating structure of n o 3 −: (i) resonating structure of ozone ( o.
Write two resonance structures of ozone which satisfy the octet rule
Web ozone, or #o_3#, has two major resonance structures that contribute equally to the overall hybrid structure of the molecule. 433k views 10 years ago. Web if there are equivalent resonance structures, draw all of them. Web the first resonance structure shown below has one positively charged oxygen, one negatively charged oxygen and one neutral oxygen with two bonds. Web.
Resonance Structures Easy Hard Science
(i) resonating structure of ozone ( o 3 ): 131k views 12 years ago every video. Web here is a diagrammatic representation of the mo diagram of ozone. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). After drawing the lewis structure of nh 3, the shape.
Ozone Lewis Structure How to Draw the Lewis Structure for Ozone YouTube
For example, drawing one lewis structure for ozone (o 3) gives us a. Web here is a diagrammatic representation of the mo diagram of ozone. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). In this post, we will be drawing the lewis structure of ozone, o.
Resonance Structures of O3, Ozone YouTube
Web this lesson walks you through each step of drawing the lewis structure of o 3. For example, drawing one lewis structure for ozone (o 3) gives us a. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). (i) resonating structure of ozone ( o 3 ):.
For Example, Drawing One Lewis Structure For Ozone (O 3) Gives Us A.
(i) resonating structure of ozone ( o 3 ): (figure 2) was this answer helpful? Ozone, o 3, an unstable, blue, diamagnetic gas with a characteristic pungent odor, protects the earth. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms).
Web A Molecule Or Ion With Such Delocalized Electrons Is Represented By Several Contributing Structures (Also Called Resonance Structures Or Canonical Forms).
After drawing the lewis structure of nh 3, the shape of the o 3 molecule can be. Such is the case for. (figure 1) (ii) resonating structure of n o 3 −: I quickly take you through how to draw the lewis structure of o3 (ozone).
We Can Draw O3 Different Ways.
For the o3 structure use the periodic table to find. In this post, we will be drawing the lewis structure of ozone, o 3. 85k views 3 years ago new ap & general chemistry video playlist. Web this lesson walks you through each step of drawing the lewis structure of o 3.
Web Here Is A Diagrammatic Representation Of The Mo Diagram Of Ozone.
Web ozone, or #o_3#, has two major resonance structures that contribute equally to the overall hybrid structure of the molecule. We're using all 18 valence electrons. Web resonance structures of o3. Web when this happens you need to draw resonance structures, none of which accurately describe the bonds, with the real structure sort of being the average of all the.


.PNG)