Draw An Energy Diagram For An Exothermic Reaction
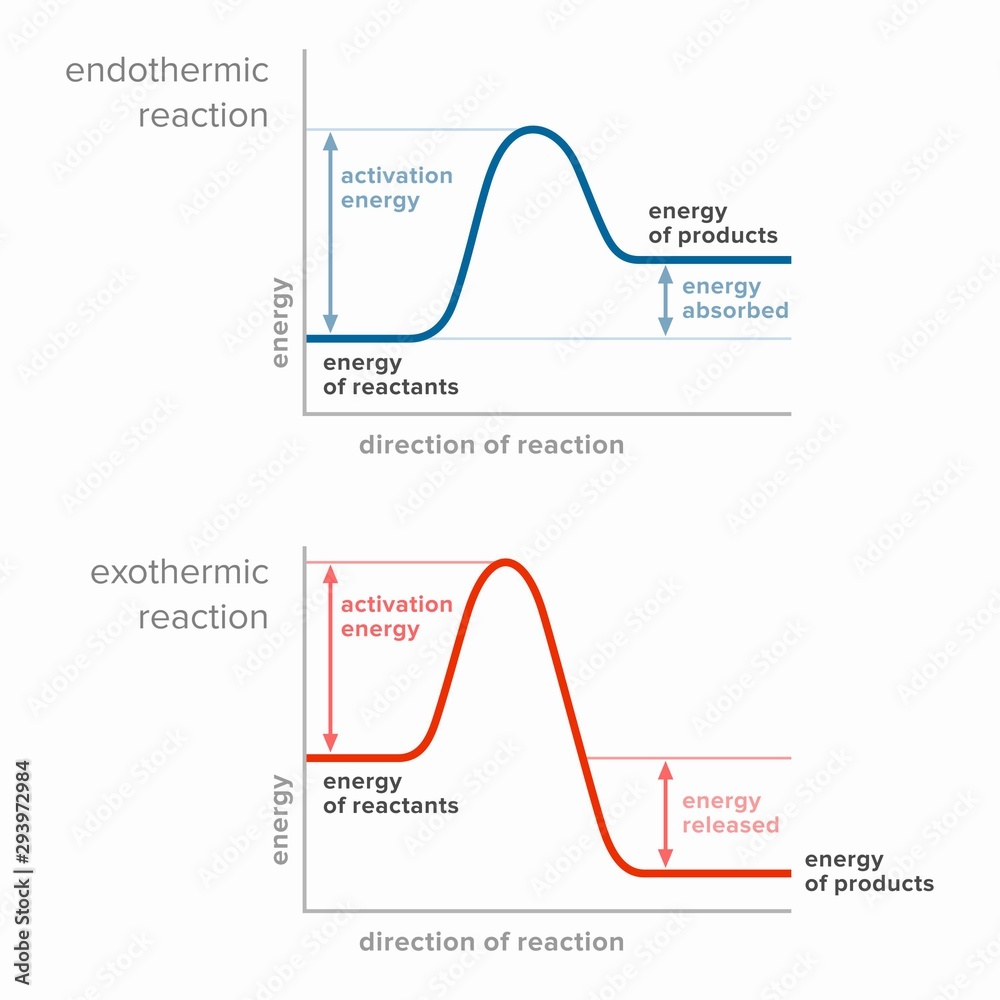
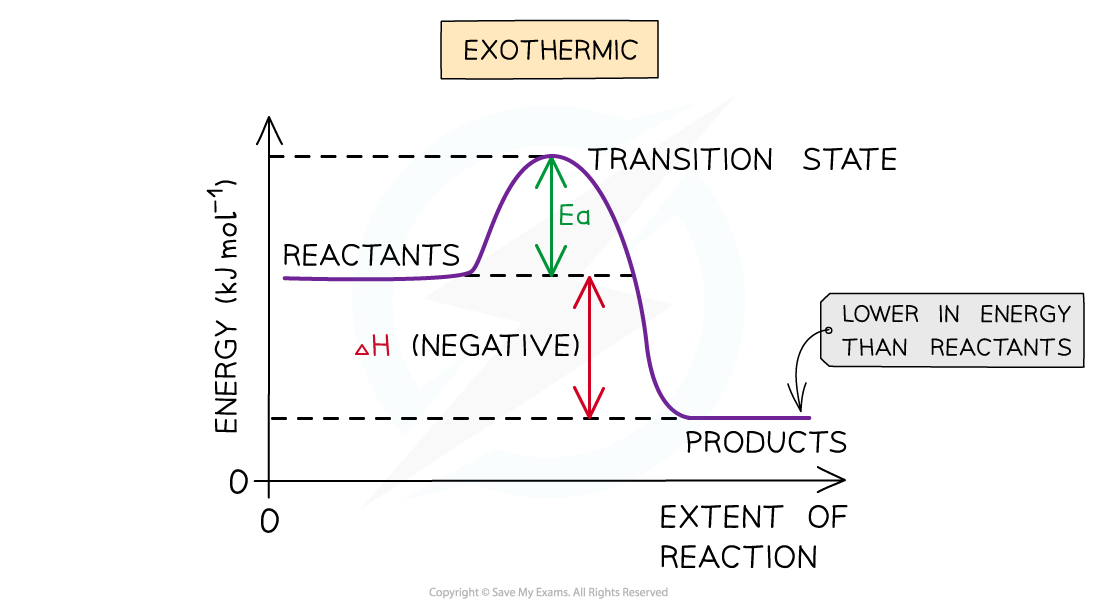
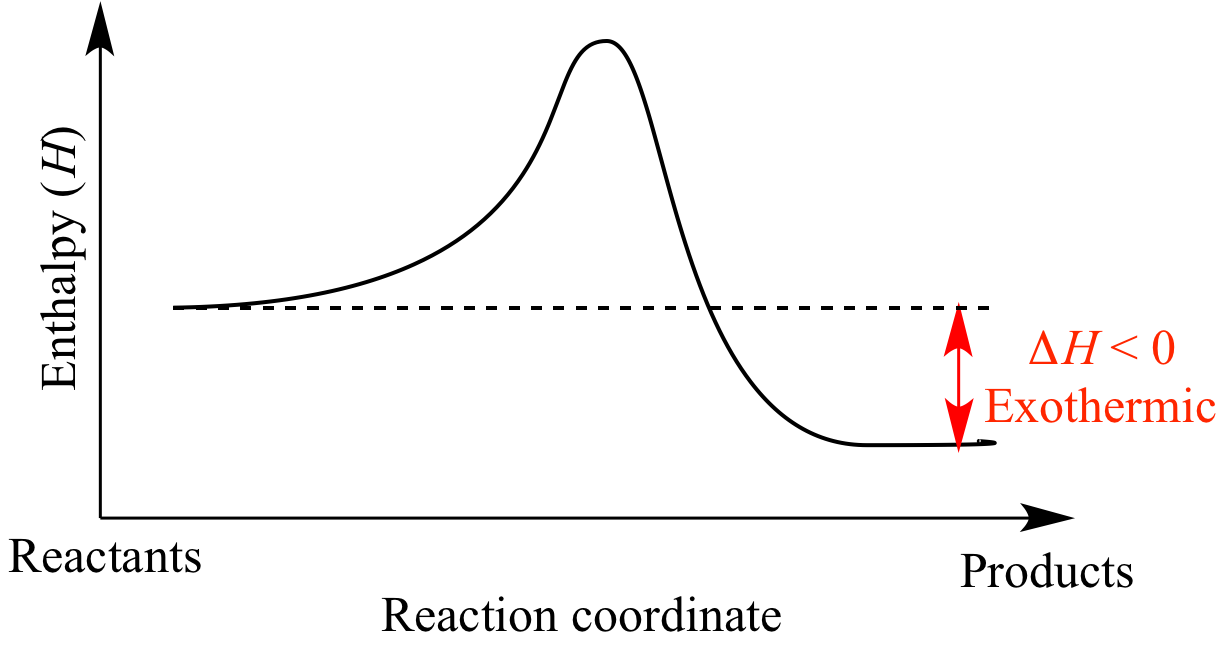
Draw An Energy Diagram For An Exothermic Reaction - Web diagrams like this are described as energy profiles. If the energy of the products is lower than the energy of the reactants, the reaction is exothermic. Web the diagram could be used to tell if the reaction is endothermic or exothermic. Web this chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams. Then, draw anothercurve corresponding to the reaction in the presence of a catalyst that reduces theactivation energy by 20kjmol. With an activation energy of 4 0 k j m o l and a δ h. The activation energy is also lower, which means that less energy is required to initiate. The chemical equation for the complete combustion of methane is: Web an energy level diagram close energy level diagram chart showing the energy in the reactants and products, and the difference in energy between them. The transition state is an ‘ activated complex’:
With an activation energy of 4 0 k j m o l and a δ h. Web helpful steps to create an energy diagram. Web chemistry questions and answers. In the energy diagram for an exothermic reaction, the potential energy of the products is lower than that of the reactants.this indicates that energy has been released from the system, as the reactants have converted to products. Web organic chemistry with a biological emphasis by tim soderberg (university of minnesota, morris) 5.6: The sign of the enthalpy change is positive or. The chemical equation for the complete combustion of methane is: So, the activation energy is the minimum amount of energy required for a. Web this chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams. Web an energy level diagram close energy level diagram chart showing the energy in the reactants and products, and the difference in energy between them.
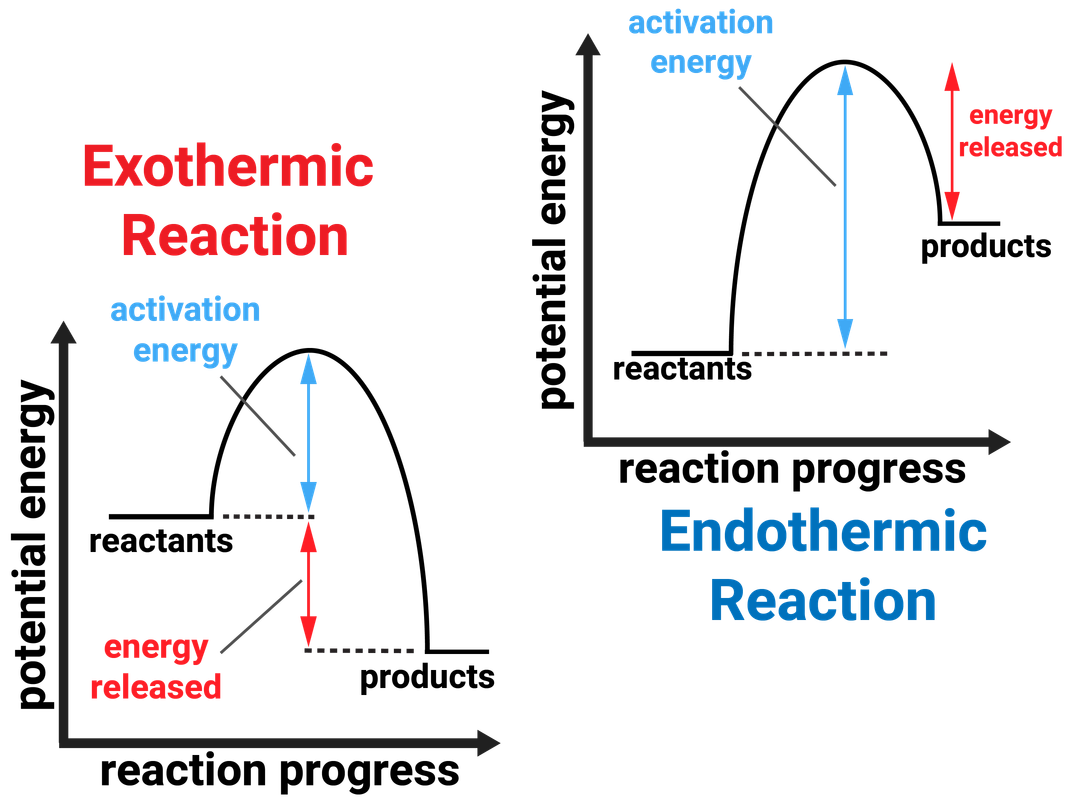
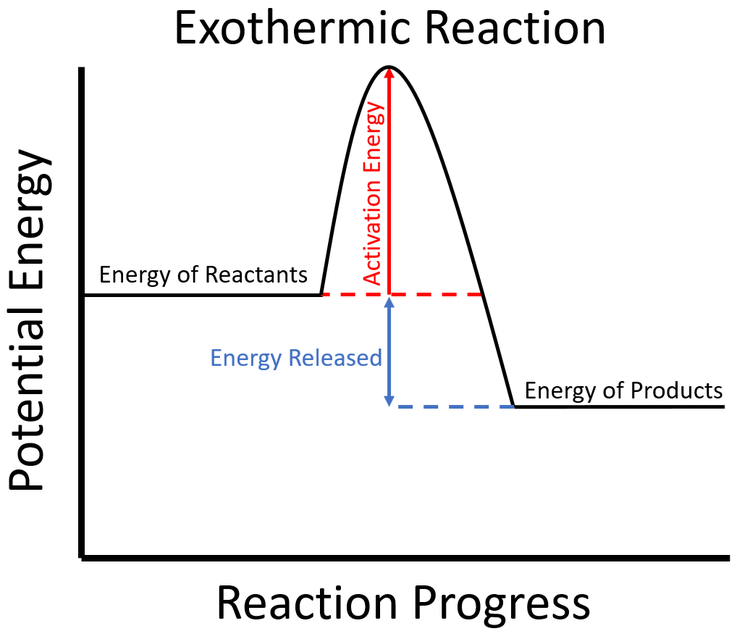
2) draw a line at the beginning of the graph for the reactants and a line at the end for the products. Web an energy level diagram close energy level diagram chart showing the energy in the reactants and products, and the difference in energy between them. In an exothermic reaction, heat is released (considered a product) and the energy of the system decreases (δ h is negative). Web diagrams like this are described as energy profiles. One can calculate the e a c t and δ h for any reaction from its energy diagram. Reaction energy diagrams efficiently and effectively communicate the thermodynamics and kinetics of chemical. In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products.strong bonds have lower potential energy than weak bonds. This video solution was recommended by our tutors as helpful for the problem above. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. Web in this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled.
Energy level diagrams Endothermic & Exothermic reactions
The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. The higher.
Activation energy in endothermic and exothermic reactions. Stock
This video solution was recommended by our tutors as helpful for the problem above. Web the diagram could be used to tell if the reaction is endothermic or exothermic. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. At the peak of the activation energy hump, the reactants.
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk
2) draw a line at the beginning of the graph for the reactants and a line at the end for the products. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. If you had an endothermic reaction, a. Depiction of an energy diagram. Web draw the energy.
Exothermic Key Stage Wiki
Web in this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. So, the activation energy is the minimum amount of energy required for a. If the energy of the products is lower than the energy of the reactants, the reaction is exothermic. Draw the potential energy diagram of an exothermic sn1 reaction..
Schematic representation of the energy level diagram of an exothermic
Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction. Web organic chemistry with a biological emphasis by tim soderberg (university of minnesota, morris) 5.6: Web this chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams. Ch4 (g) + 2o2 (g) → co2.
Energy Diagram — Overview & Parts Expii
This state is also known as an activated complex. Depiction of an energy diagram. A potential energy diagram for an s n 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition.
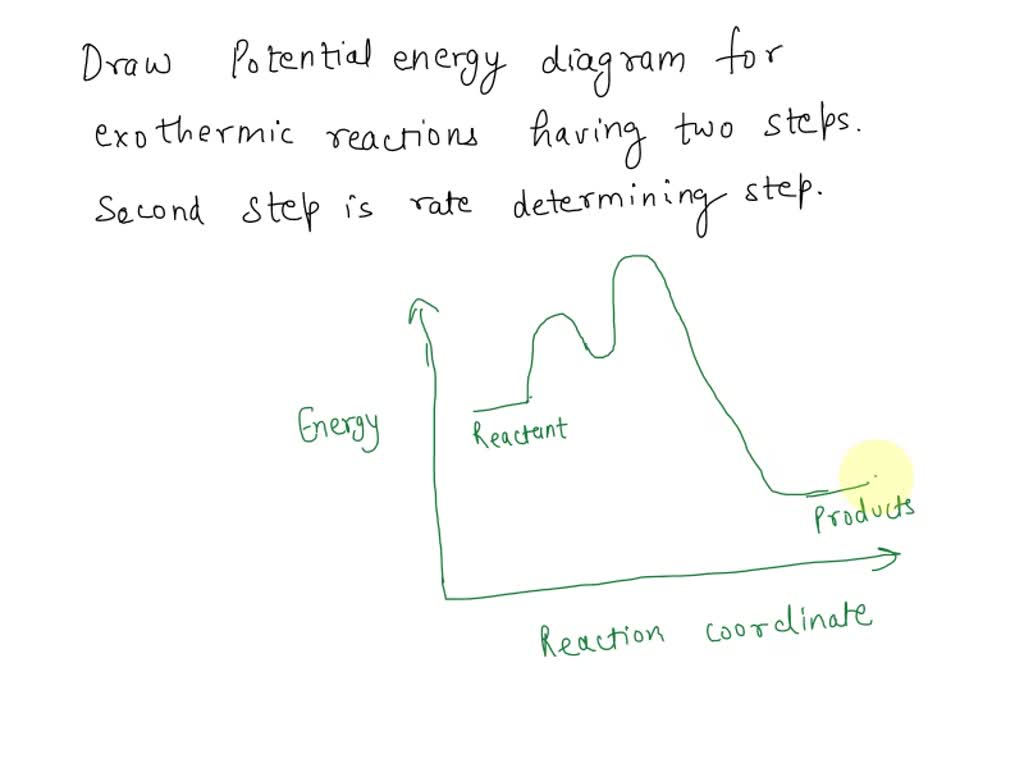
SOLVED Draw the energy diagram for a twostep exothermic reaction
In an exothermic reaction, heat is released (considered a product) and the energy of the system decreases (δ h is negative). Web in the s n 1 reaction, the carbocation species is a reaction intermediate. Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction. Shows whether a reaction is exothermic close. A potential.
How to draw Energy Profile Diagram and Energy Level Diagram of
It also shows the effect of a catalyst on the f. With an activation energy of 4 0 k j m o l and a δ h. Then, draw anothercurve corresponding to the reaction in the presence of a catalyst that reduces theactivation energy by 20kjmol. They compare the energy of the reactants to the energy of the products. Web.
CIE A Level Chemistry复习笔记1.5.2 Energy Level Diagrams翰林国际教育
Web helpful steps to create an energy diagram. At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in the process of breaking and forming. In the diagram above, you can clearly see that you need an input of energy to get the reaction going..
Illustrated Glossary of Organic Chemistry Exothermic
In the diagram above, you can clearly see that you need an input of energy to get the reaction going. 2) draw a line at the beginning of the graph for the reactants and a line at the end for the products. Web the diagram could be used to tell if the reaction is endothermic or exothermic. Reaction energy diagrams.
Then, Draw Anothercurve Corresponding To The Reaction In The Presence Of A Catalyst That Reduces Theactivation Energy By 20Kjmol.
The sign of the enthalpy change is positive or. Web in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Draw the curve in the energy. So, the activation energy is the minimum amount of energy required for a.
Ch4 (G) + 2O2 (G) → Co2 (G) + 2H2O (L) Step 2:
Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. This state is also known as an activated complex. At the very top of the energy barrier, the reaction is at its transition state (ts), which is the point at which the bonds are in the process of breaking and forming. Activation energy graph for co.
Web Diagrams Like This Are Described As Energy Profiles.
One can calculate the e a c t and δ h for any reaction from its energy diagram. The higher the energy hill, the slower the reaction. Web helpful steps to create an energy diagram. Web the total potential energy of the system decreases for the exothermic reaction as the system releases energy to the surroundings.
At The Peak Of The Activation Energy Hump, The Reactants Are In The Transition State, Halfway Between Being Reactants And Forming Products.
Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: Web this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Web organic chemistry with a biological emphasis by tim soderberg (university of minnesota, morris) 5.6: To depict graphically the energy changes that occur during a reaction, chemists use energy diagrams, such as that in figure 6.5.the vertical axis of the diagram.