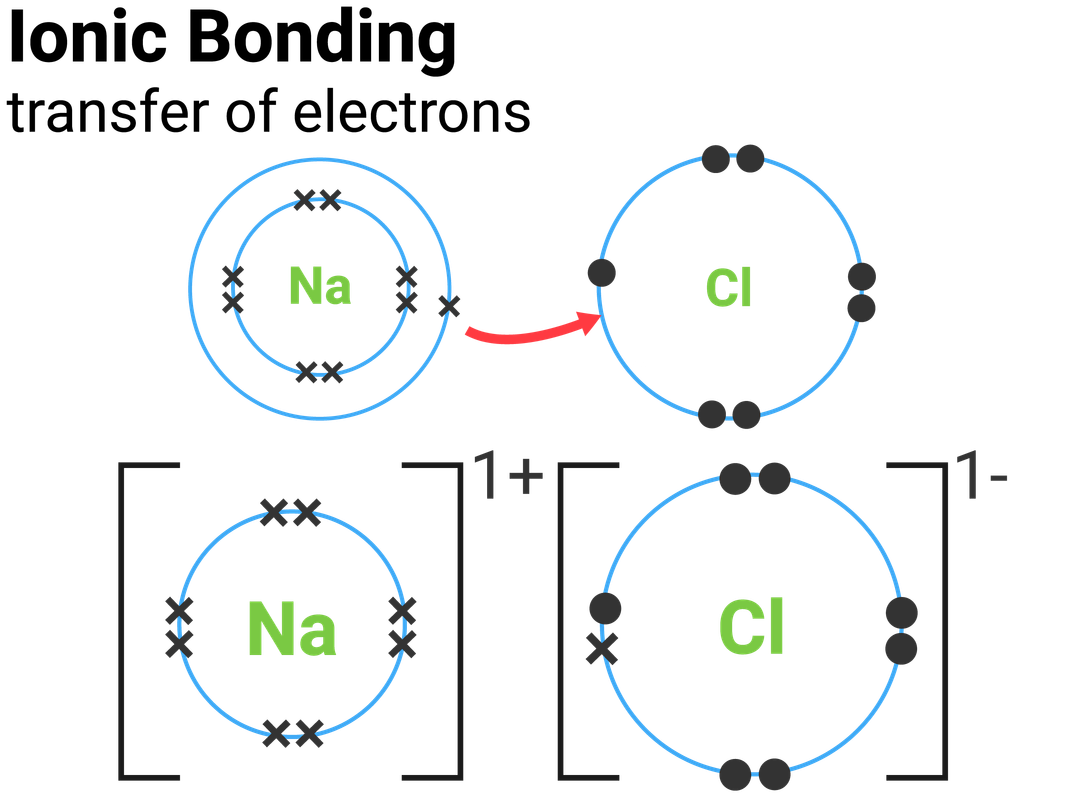
Draw An Ionic Bond
Draw An Ionic Bond - Ionic bonds are caused by electrons transferring from one atom to another. Show how electrons are transferred in ionic bonding. Web they're nice ways of visualizing how the atoms in a molecule are bonded to each other and what other lone pairs of valence electrons various atoms might have. State the limitations of a range of models used to represent ionic bonding. The slideshow shows ionic bonds being formed in sodium chloride, magnesium oxide, calcium chloride. The octet rule refers to the tendency of atoms to. The energy of the electrostatic attraction ( e ), a measure of the force’s strength, is inversely proportional to the internuclear distance between the charged particles (. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. This is because valence electrons.
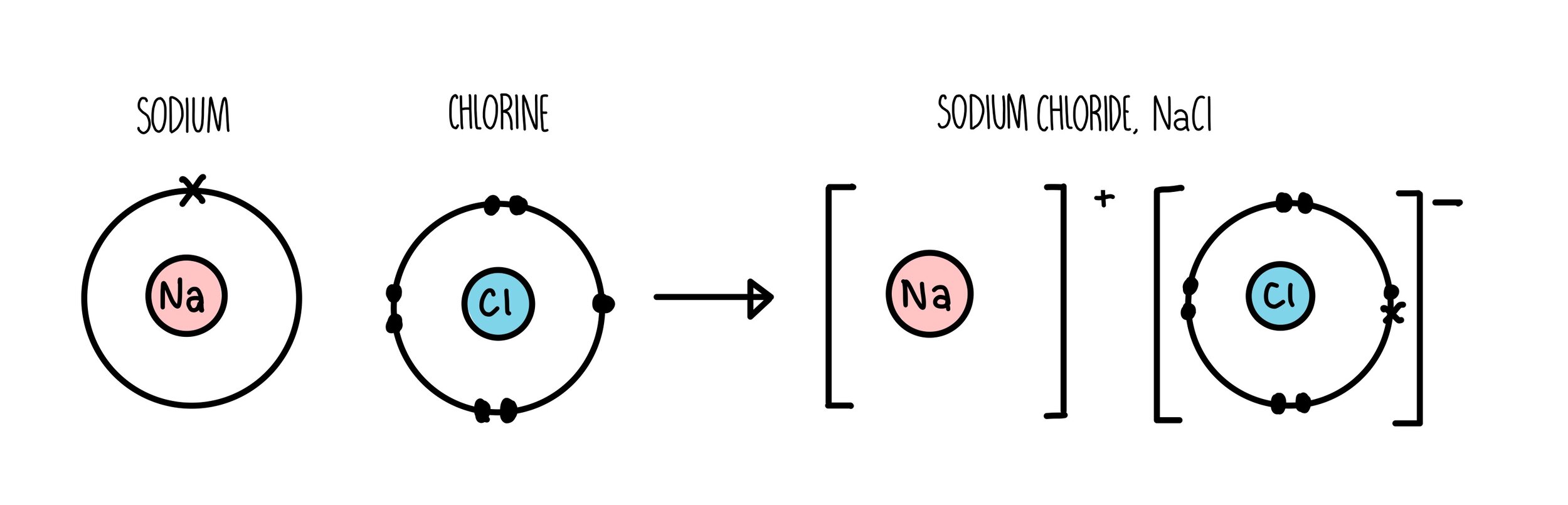
Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. Web drawing lewis dot structures (lds) ionic ldss. (note that we denote ions with brackets around the structure, indicating the. First, write the empirical formula of the compound down to see which elements are involved and how many atoms of. Web shows how to draw lewis dot structures for ionic compounds. This is because valence electrons. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Web when drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular compounds. Introduction activities 1 and 2 introduce ionic bonding dot and cross diagrams in a format that A dot and cross diagram is one way to model the transfer of electrons that occurs during this process.
An ionic bond forms between a metal and a nonmetal. The energy of the electrostatic attraction ( e ), a measure of the force’s strength, is inversely proportional to the internuclear distance between the charged particles (. Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Draw lewis structures for ionic compounds. Web how to draw ionic bonds from education in chemistry which can be viewed at rsc.li/3amzz9j learning objectives 1 draw dot and cross diagrams for ionic compounds. Web this chemistry video explains how to draw the lewis structures of ionic compounds. State the limitations of a range of models used to represent ionic bonding. Ionic bonds are caused by electrons transferring from one atom to another.
Ionic bond Science, Chemistry, Chemical Bonds ShowMe
Web draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Introduction activities 1 and 2 introduce ionic bonding dot and cross diagrams in a format that Show how electrons are transferred in ionic.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Introduction activities 1 and 2 introduce ionic bonding dot and cross diagrams in a format that First, write the empirical formula of the compound down to see which elements are involved and how many atoms of. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in.
Ionic Bonds Dot & Cross Diagrams (1.6 4) Edexcel IGCSE Chemistry
An ionic bond forms between a metal and a nonmetal. Ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Once they have mastered electron configuration diagrams, show your learners how they can adapt them.
Ionic Bonding — the science hive
Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Draw lewis structures for ionic compounds. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. The octet rule.
Drawing Ionic Bonds YouTube
Web drawing lewis dot structures (lds) ionic ldss. The energy of the electrostatic attraction ( e ), a measure of the force’s strength, is inversely proportional to the internuclear distance between the charged particles (. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry..
Examples of Ionic Bonds and Compounds
First, write the empirical formula of the compound down to see which elements are involved and how many atoms of. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. Web drawing lewis dot.
Ionic Bonding Presentation Chemistry
Web the transfer of electrons in order to achieve a noble gas configuration is the process known as ionic bonding, and this will be covered in more detail later in this chapter. Web drawing lewis dot structures (lds) ionic ldss. 6.7k views 7 years ago edexcel. Web how to draw ionic bonds from education in chemistry which can be viewed.
ionic bond Definition, Properties, Examples, & Facts Britannica
Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another. Web drawing lewis dot structures (lds) ionic ldss. Web they're nice ways of visualizing how the atoms in a molecule are bonded to.
Ionic Bonding Diagram Amashusho Images
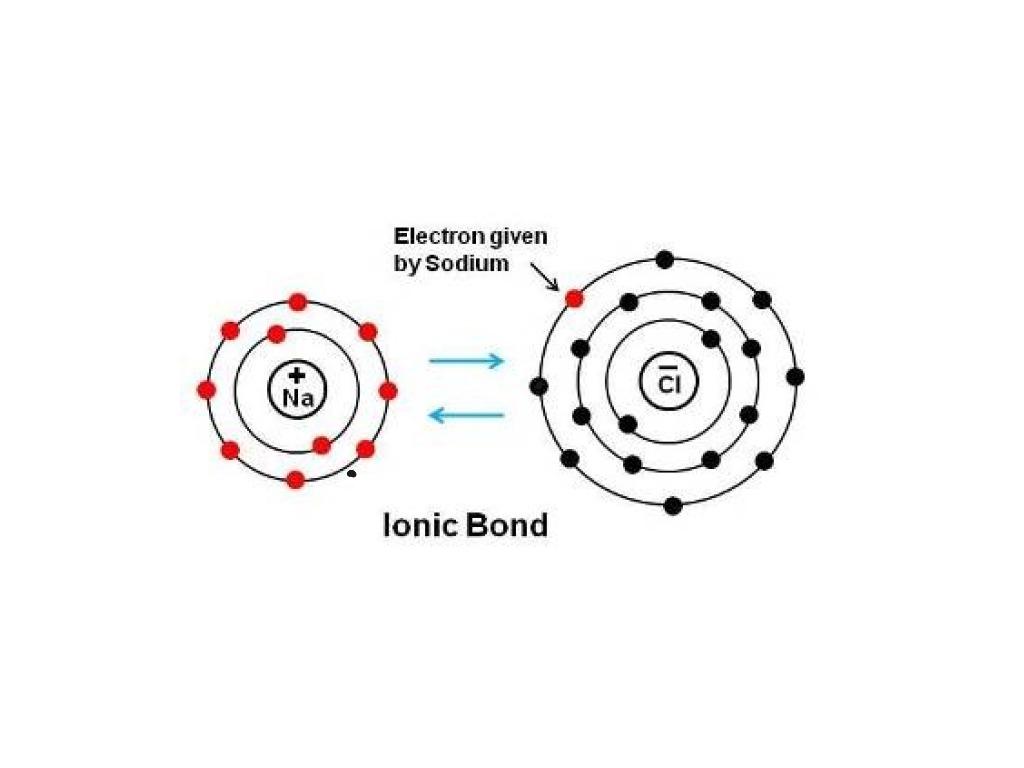
Web one electron will be transferred from the outer shell of the sodium atom to the outer shell of the chlorine atom. The octet rule refers to the tendency of atoms to. Show how electrons are transferred in ionic bonding. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web the attraction between oppositely charged.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. Ionic bonds are caused by electrons transferring from one atom to another. Web they're nice ways of visualizing how the atoms in a molecule are bonded to each other and what other lone pairs of.
Draw Lewis Structures For Ionic Compounds.
Introduction activities 1 and 2 introduce ionic bonding dot and cross diagrams in a format that The energy of the electrostatic attraction ( e ), a measure of the force’s strength, is inversely proportional to the internuclear distance between the charged particles (. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together.
Web One Electron Will Be Transferred From The Outer Shell Of The Sodium Atom To The Outer Shell Of The Chlorine Atom.
An ionic bond forms between a metal and a nonmetal. Web shows how to draw lewis dot structures for ionic compounds. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces.
Web When Drawing Lewis Dot Structures For Ionic Compounds You Need To Follow A Different Set Of Rules Than With Lewis Structures For Covalent/Molecular Compounds.
Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds. Ionic bonds are caused by electrons transferring from one atom to another. Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. Web the attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry.
First, Write The Empirical Formula Of The Compound Down To See Which Elements Are Involved And How Many Atoms Of.
Web drawing lewis dot structures (lds) ionic ldss. 6.7k views 7 years ago edexcel. The slideshow shows ionic bonds being formed in sodium chloride, magnesium oxide, calcium chloride. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process.



.PNG)