Draw Lewis Structure For Cn
Draw Lewis Structure For Cn - The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Include all lone pairs of electrons and nonbonding electrons. Molecular shapes & valence bond theory mo theory: Web drawing lewis structures for molecules with one central atom: #2 mark lone pairs on the atoms. Web this widget gets the lewis structure of chemical compounds. Write lewis symbols for neutral atoms and ions. Web we can draw lewis structures for polyatomic ions (ions containing multiple atoms) using the same stepwise procedure as for neutral molecules.
#1 draw a rough sketch. Let’s break down each step in detail. Shared pairs of electrons are drawn as lines between atoms, while. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. The carbon atom and nitrogen atom, both have one lone pair. Using lewis structures to show valence electrons. Web draw lewis structures depicting the bonding in simple molecules. Write lewis symbols for neutral atoms and ions. Do bonding between all the elements present in structure. Include all lone pairs of electrons and nonbonding electrons.
Let’s draw and understand this lewis dot structure step by step. #5 if central atom doesn’t form octet, convert lone pair and mark charges again. #3 mark charges on the atoms. 4.7k views 11 months ago. #4 minimize charges by converting lone pairs. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Calculation of total valence electrons present on structure. Here since an oxygen atom has an atomic number of 8, we have six electrons in the outermost shell. By the end of this section, you will be able to: Molecular shapes & valence bond theory mo theory:
CN Lewis Structure (Cyanide) YouTube
Here since an oxygen atom has an atomic number of 8, we have six electrons in the outermost shell. Want to join the conversation? Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up.
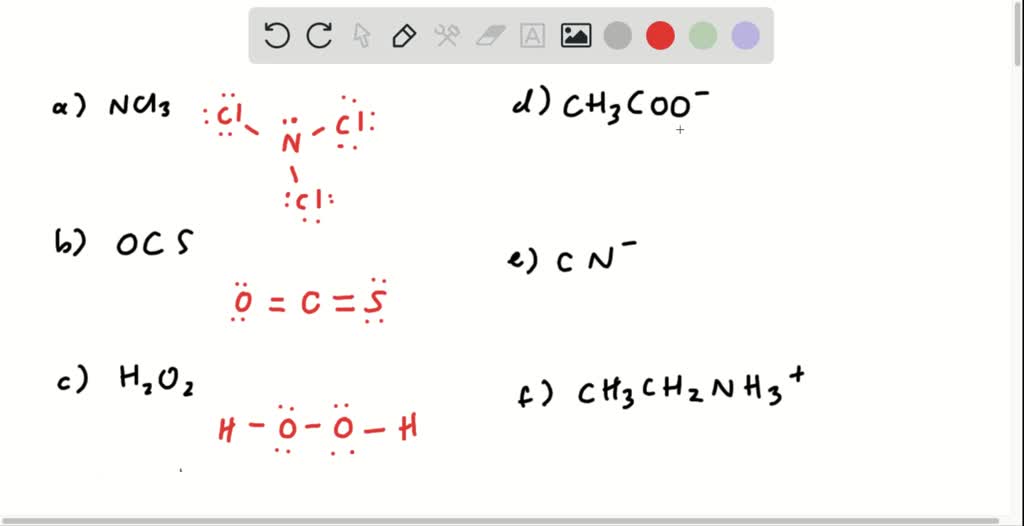
SOLVED 3) Draw Lewis structure for each them) (draw
Shared pairs of electrons are drawn as lines between atoms, while. Let’s draw and understand this lewis dot structure step by step. I also go over the. Using the periodic table to draw lewis dot structures. #1 draw a rough sketch.
CN Lewis Structure Lewis Dot Structure for CNCyanide Ion Lewis
The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. According to the lewis model, which species. Write lewis symbols for neutral atoms and ions. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on. In this video,.
How do you draw the Lewis structure for CN ? Lewis Dot Structure of
Select the element with lowest electronegativity for central position in structure. Write lewis symbols for neutral atoms and ions. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on. 158k views 12 years ago every video. Draw lewis structures for molecules.
CN Lewis Structure How to Draw the Dot Structure for the CN YouTube
Shared pairs of electrons are drawn as lines between atoms, while. #1 draw a rough sketch. Select the element with lowest electronegativity for central position in structure. How to draw a lewis structure. By the end of this section, you will be able to:
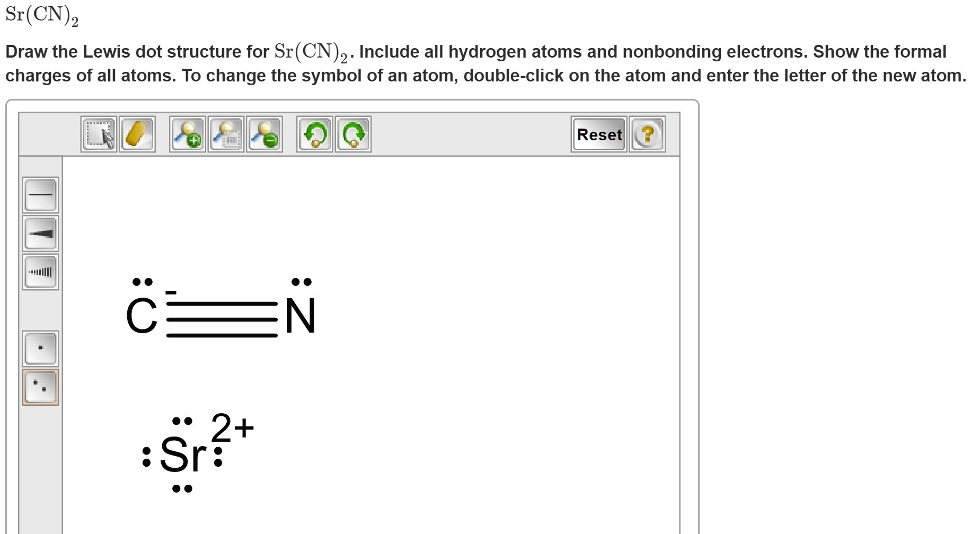
Sr(CN)2 Draw the Lewis dot structure for Sr(CN)z. Inc… SolvedLib
How to draw a lewis structure. Show the formal charges of all nonhydrogen atoms, or use square brackets to denote the overall charge. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. According to the lewis model, which species. A lewis diagram shows how the.
DRAWING LEWIS STRUCTURE YouTube
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Want to join the conversation? Using lewis structures to show valence electrons. In this video, we'll see how to construct the lewis diagram of the cyanide ion (cn⁻). Writing lewis structures with the octet rule.
CN Lewis Structure (Cyanide ion) YouTube
#2 mark lone pairs on the atoms. Draw lewis structure for cn+cn+. Write lewis symbols for neutral atoms and ions. #3 mark charges on the atoms. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.
Cn Lewis Structure Formal Charge Draw Easy
There aren't enough valence electrons available for each atom to obtain an octet without sharing more than one pair. According to the lewis model, which species. Want to join the conversation? Using the periodic table to draw lewis dot structures. Select the element with lowest electronegativity for central position in structure.
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Calculation of total valence electrons present on structure. Thus far in this chapter, we have discussed the various types of.
How To Draw A Lewis Structure.
In this video, we'll see how to construct the lewis diagram of the cyanide ion (cn⁻). #4 minimize charges by converting lone pairs. #1 draw a rough sketch. Draw the molecule by placing atoms on the grid and connecting them with bonds.
Using Lewis Structures To Show Valence Electrons.
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Writing lewis structures with the octet rule. Draw lewis structures depicting the bonding in simple molecules.
Show The Formal Charges Of All Nonhydrogen Atoms, Or Use Square Brackets To Denote The Overall Charge.
Web we can draw lewis structures for polyatomic ions (ions containing multiple atoms) using the same stepwise procedure as for neutral molecules. By the end of this section, you will be able to: Web drawing lewis structures for molecules with one central atom: #2 mark lone pairs on the atoms.
By The End Of This Section, You Will Be Able To:
In this video we'll draw the lewis. Draw lewis structure for cn+cn+. Shared pairs of electrons are drawn as lines between atoms, while. #3 mark charges on the atoms.



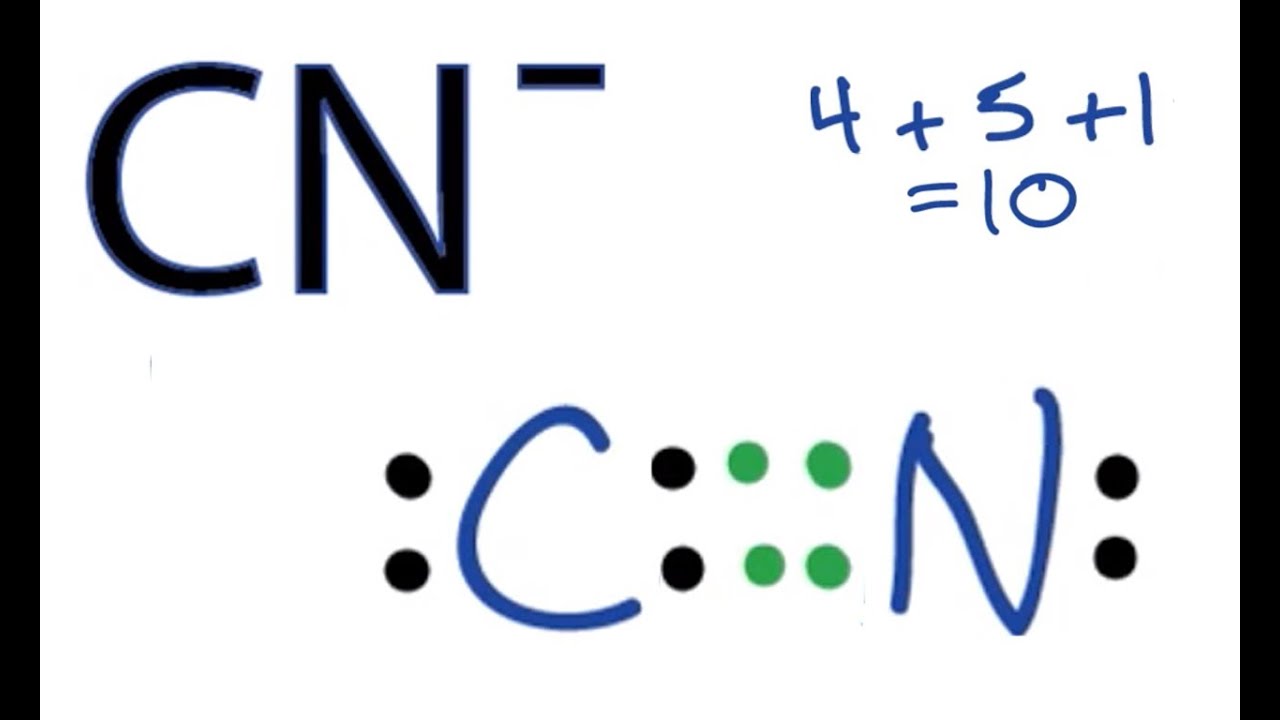


.PNG)
