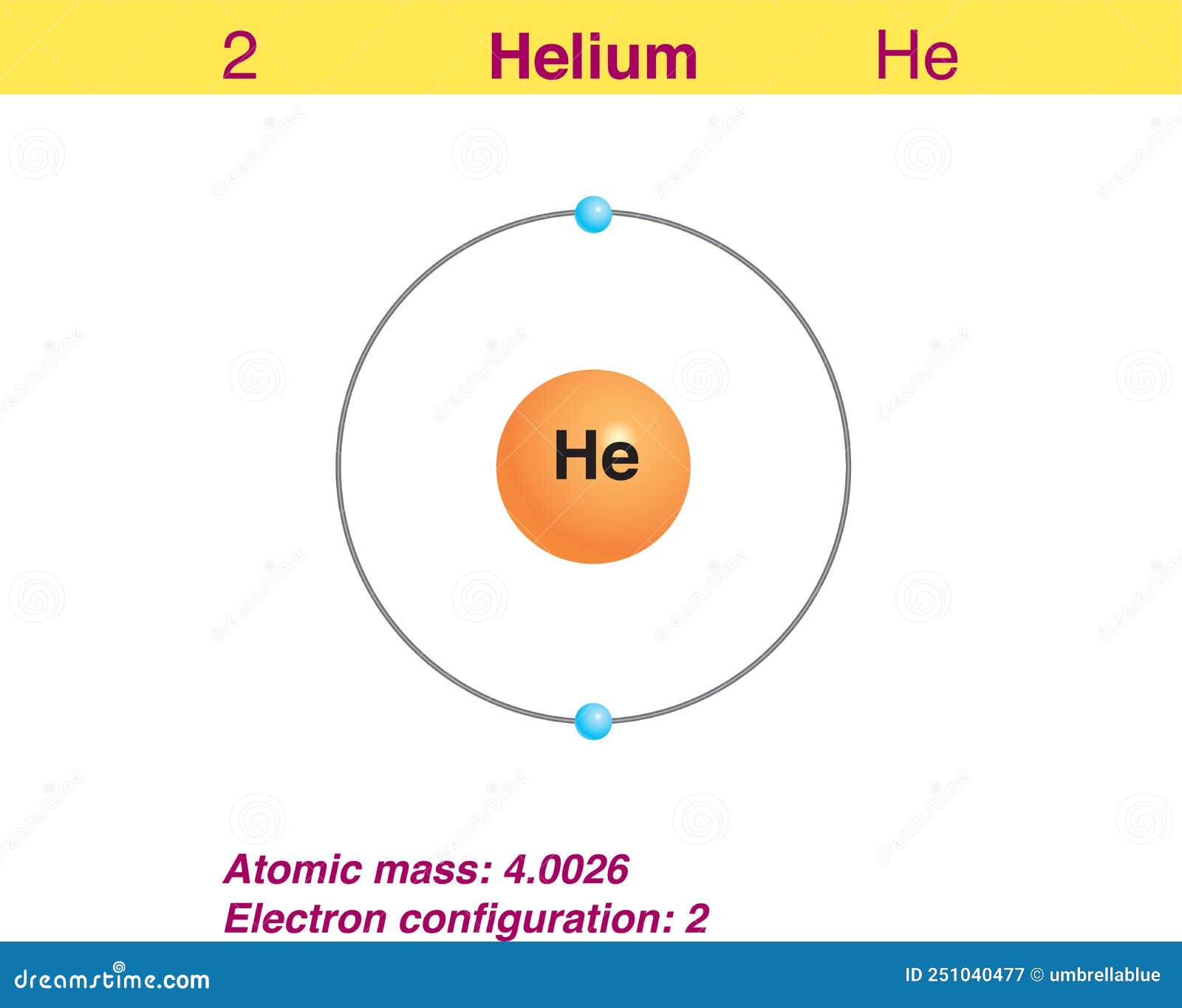
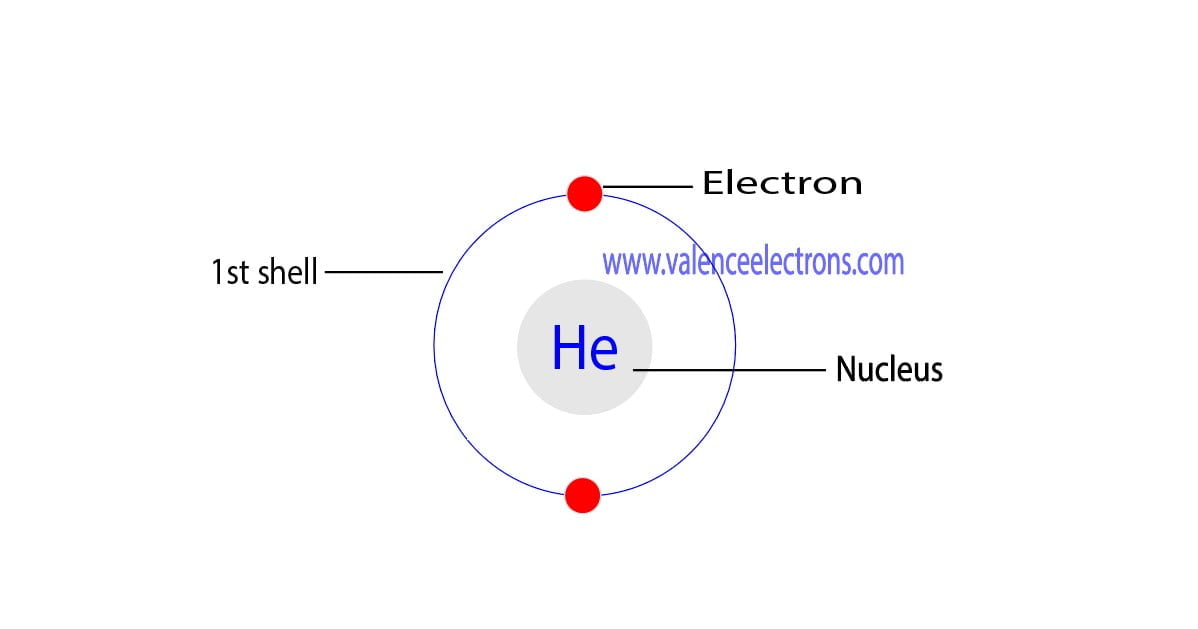
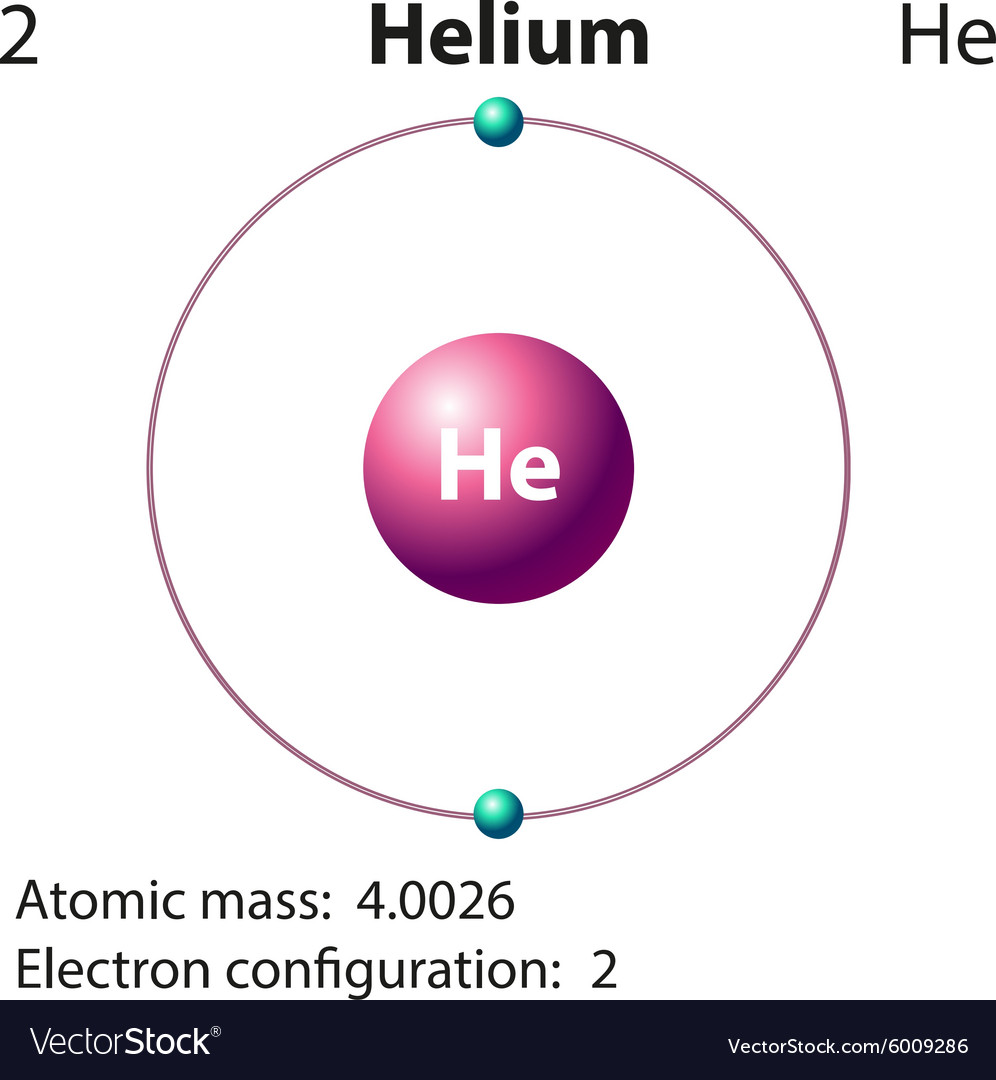
Draw The Electron Configuration For A Neutral Atom Of Helium
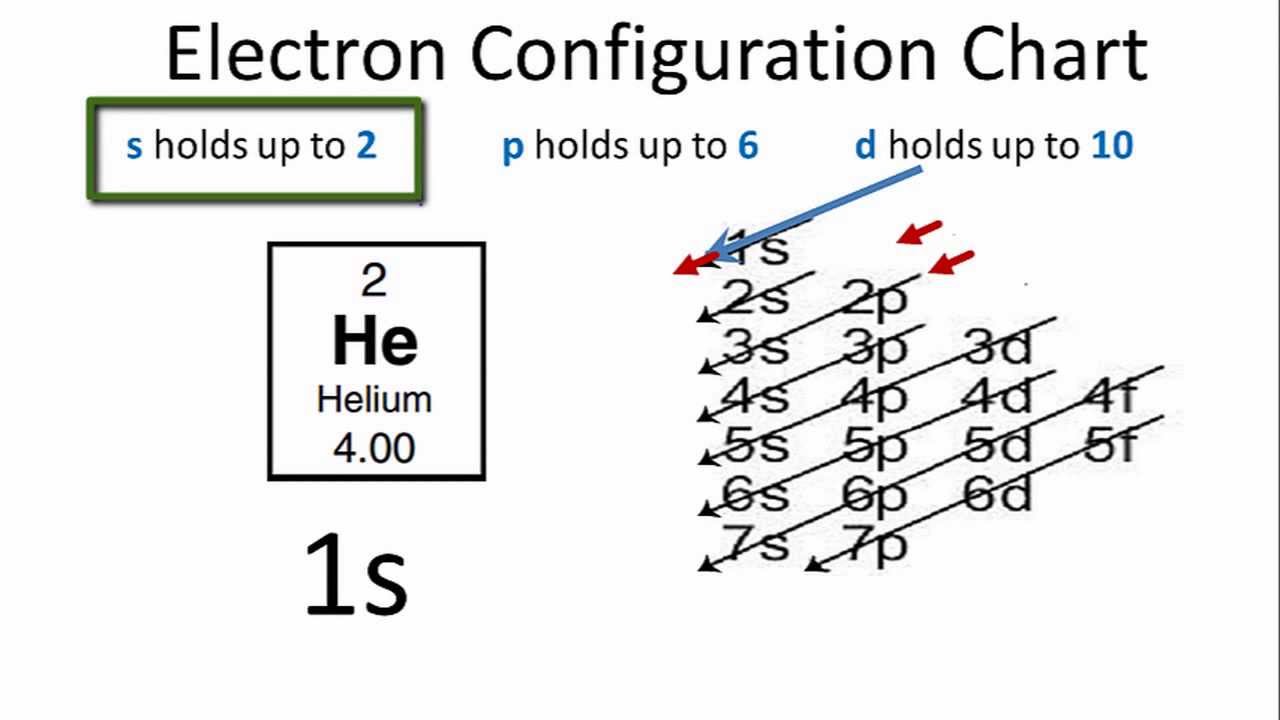
Draw The Electron Configuration For A Neutral Atom Of Helium - Enerov draw the electron configuration for a neutral atom of helium. The helium atom contains two protons and. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 2.7.1 2.7. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. Web examples of some neutral atoms and their electron configurations are shown below. Web the electron configuration and the orbital diagram are: The atomic number of helium is 2, which means it has 2 electrons. Web intro to electron configurations; Energy 1 i x $ ? Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows:
For helium, because this is the. Therefore its electron configuration is, 1s2. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 2.7.1 2.7. Web the electron configuration and the orbital diagram are: Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. Each element has a full 1s2 shell. Helium has 2 valence electrons, located in period 1, group. Web intro to electron configurations; Well what is the valence configuration of each element. Energy 1 i x $ ?
1 ), and the electron configuration is. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Following hydrogen is the noble gas helium, which has an atomic number of 2. Helium has 2 valence electrons, located in period 1, group. Electron configuration of lithium (li). Enerov draw the electron configuration for a neutral atom of helium. The helium atom contains two protons and. The first energy level (n=1) can hold a maximum of 2 electrons. You'll get a detailed solution. Well what is the valence configuration of each element.
How To Find the Helium Electron Configuration (He)
Web by kirsty patterson 6 september 2021. Using only the periodic table; For helium, because this is the. Enerov draw the electron configuration for a neutral atom of helium. Both electrons fit into the 1s subshell because s subshells.
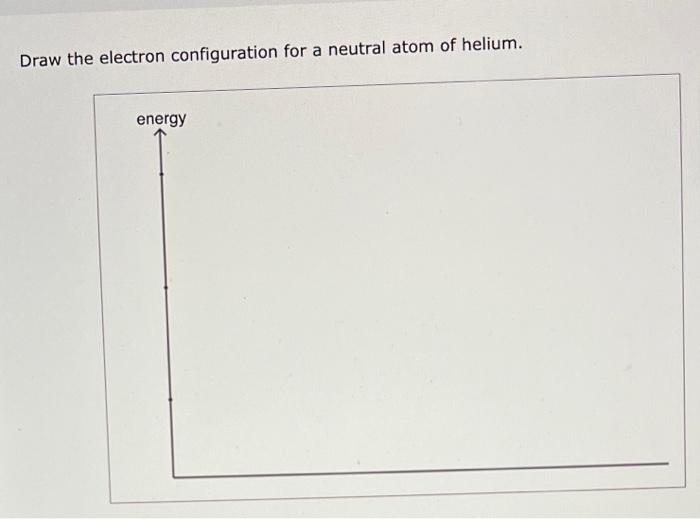
Solved Draw the electron configuration for a neutral atom of
The helium atom contains two protons and. Draw the electron configuration diagram for helium? Web electron configuration of hydrogen (h) 1s 1: Energy 1 i x $ ? This problem has been solved!
Helium, atomic structure Stock Image C018/3683 Science Photo Library
1 ), and the electron configuration is. Each element has a full 1s2 shell. Web the electron configuration and the orbital diagram are: The helium atom contains two protons and. The atomic number of helium is 2, which means it has 2 electrons.
Helium electric configuration saildop
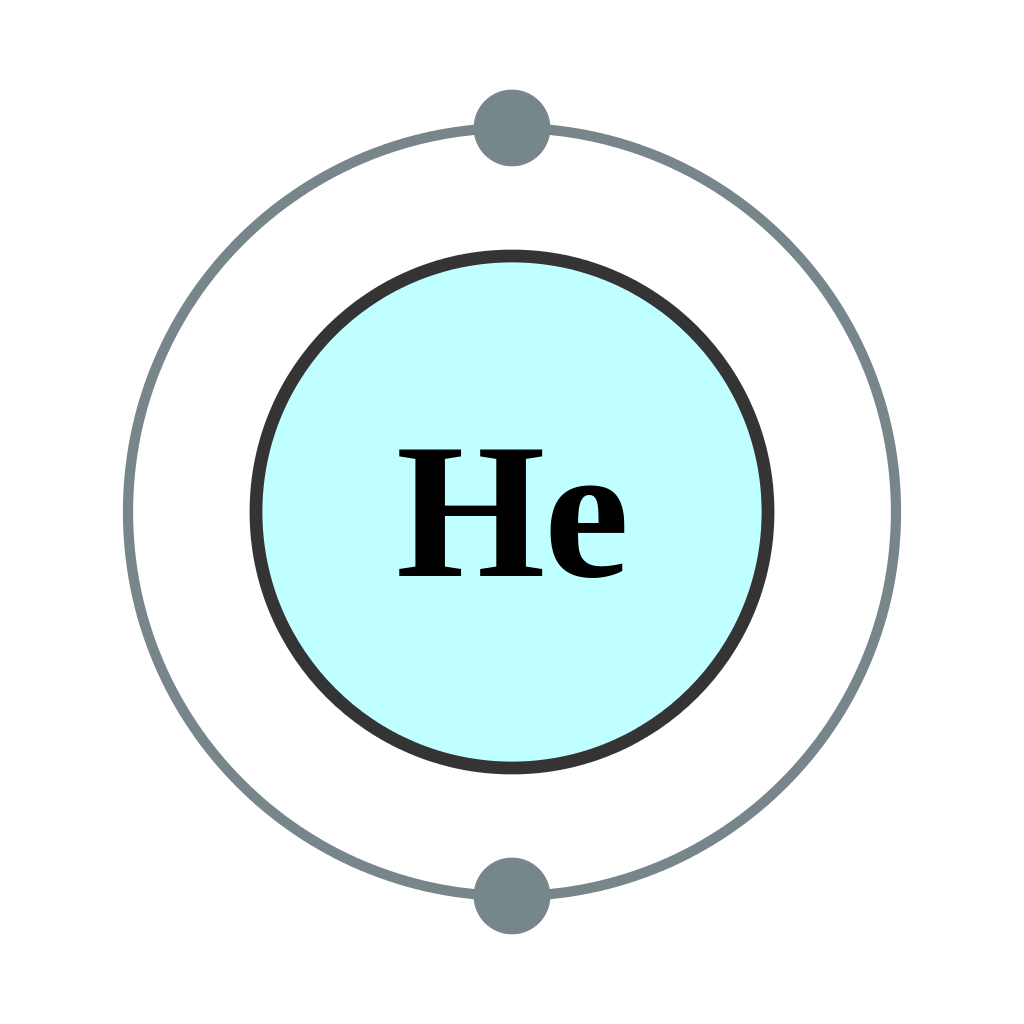
Electron configuration of lithium (li). In this table, you can see that helium has a full valence shell, with two electrons in its first and. Enerov draw the electron configuration for a neutral atom of helium. This problem has been solved! We place one electron in the orbital that is lowest in energy, the 1 s orbital.
Periodic Table Helium Protons Periodic Table Timeline
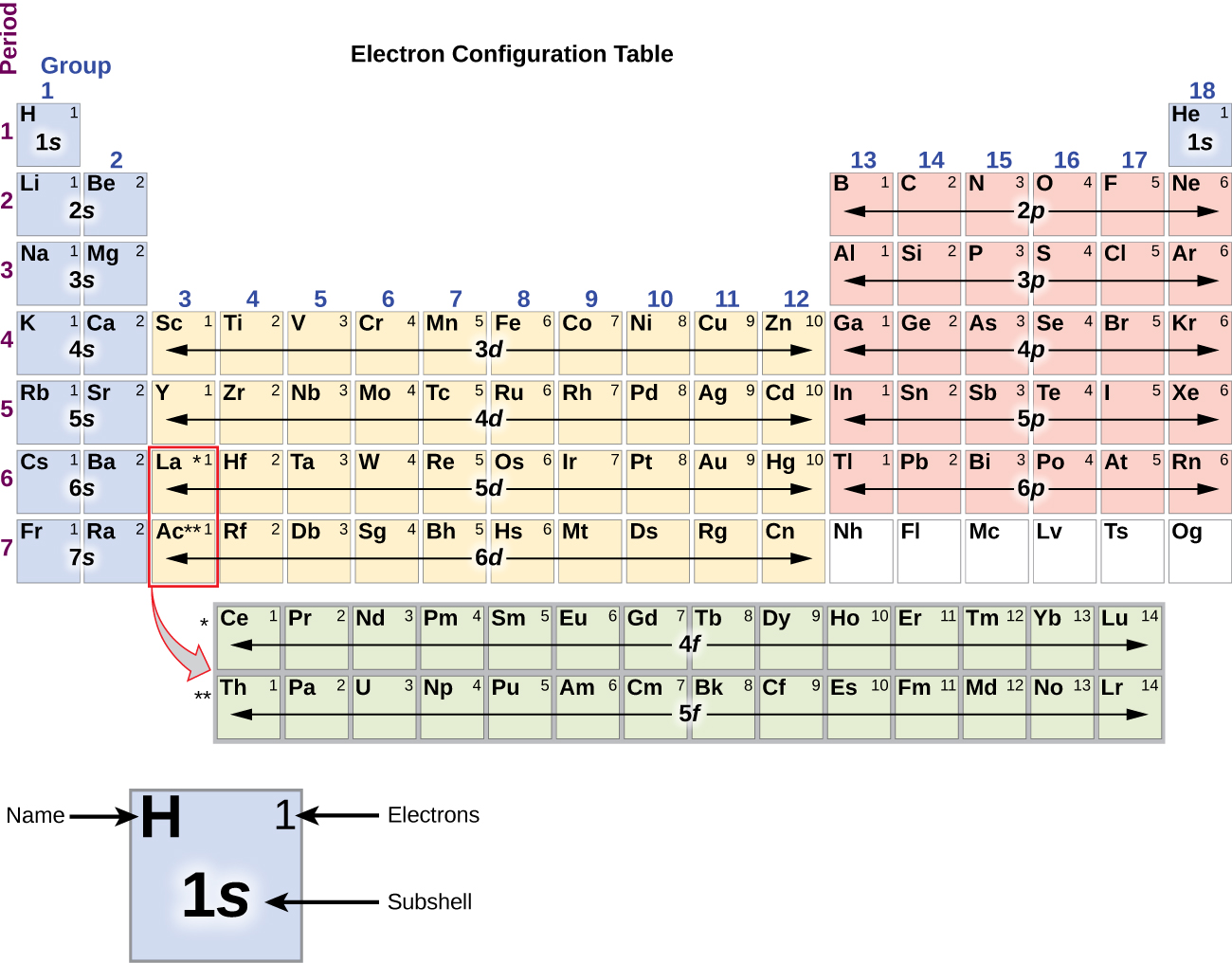
C we obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore. Web the electron configuration and the orbital diagram are: Web intro to electron configurations; In this table, you can see that helium has a full valence shell, with two electrons in its first and. Web electron configuration of hydrogen (h) 1s.
How To Find the Helium Electron Configuration (He)
Web this structure is called an electron configuration and is unique to hydrogen. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 2.7.1 2.7. Helium atoms have 2 electrons. We place one electron in the orbital that is lowest in energy, the 1 s orbital. You'll get.
Electron Arrangement in Atoms CK12 Foundation
Web examples of some neutral atoms and their electron configurations are shown below. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. Well what is the valence configuration of each element. C we obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore. Draw the.
Electron of the Element Helium Stock Vector Illustration of electron
Using only the periodic table; The first energy level (n=1) can hold a maximum of 2 electrons. Following hydrogen is the noble gas helium, which has an atomic number of 2. For helium, because this is the. C we obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore.
Electron Configuration Of Helium
The helium atom contains two protons and. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 2.7.1 2.7. Each element has a full 1s2 shell. Web this structure is called an electron configuration and is unique to hydrogen. Energy 1 i x $ ?
Diagram representation of the element helium Vector Image
Electron configuration of lithium (li). Web examples of some neutral atoms and their electron configurations are shown below. Following hydrogen is the noble gas helium, which has an atomic number of 2. Web the electron configuration and the orbital diagram are: Draw the electron configuration diagram for helium?
Electron Configuration Of Lithium (Li).
C we obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. Web electron configuration of hydrogen (h) 1s 1: In this table, you can see that helium has a full valence shell, with two electrons in its first and.
Web The Electron Configuration And The Orbital Diagram Are:
The helium atom contains two protons and. Both electrons fit into the 1s subshell because s subshells. The helium atom contains two protons and. We place one electron in the orbital that is lowest in energy, the 1 s orbital.
Web Intro To Electron Configurations;
Following hydrogen is the noble gas helium, which has an atomic number of 2. Enerov draw the electron configuration for a neutral atom of helium. Draw the electron configuration diagram for helium? Web examples of some neutral atoms and their electron configurations are shown below.
Web To Write The Electron Configuration Of An Atom, Identify The Energy Level Of Interest And Write The Number Of Electrons In The Energy Level As Its Superscript As Follows:
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Following hydrogen is the noble gas helium, which has an atomic number of 2. Therefore its electron configuration is, 1s2. Energy 1 i x $ ?