Draw The Electron Configuration For A Neutral Atom Of Scandium
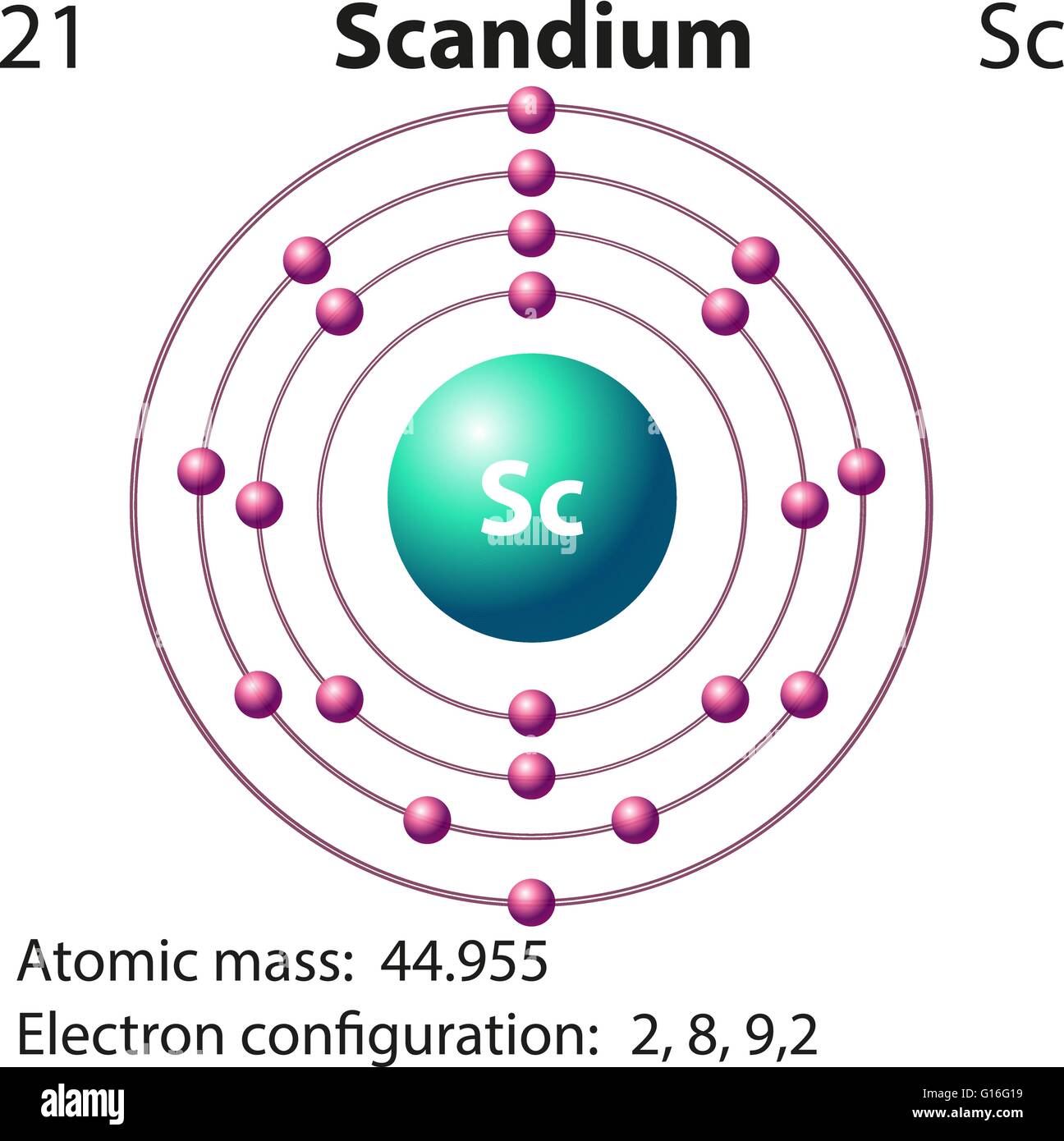
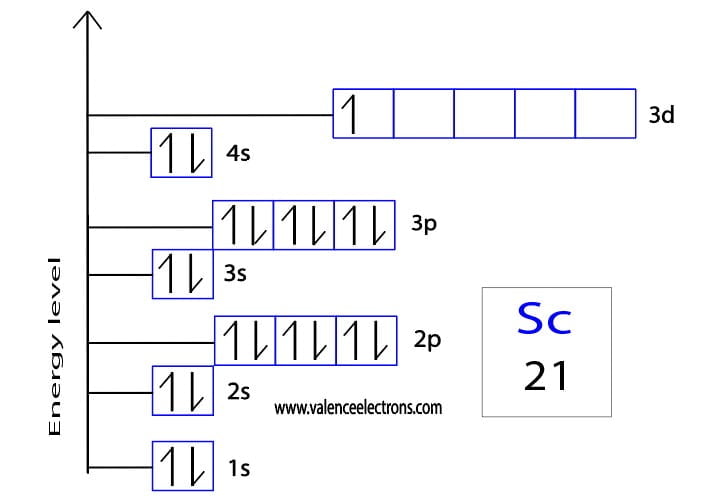
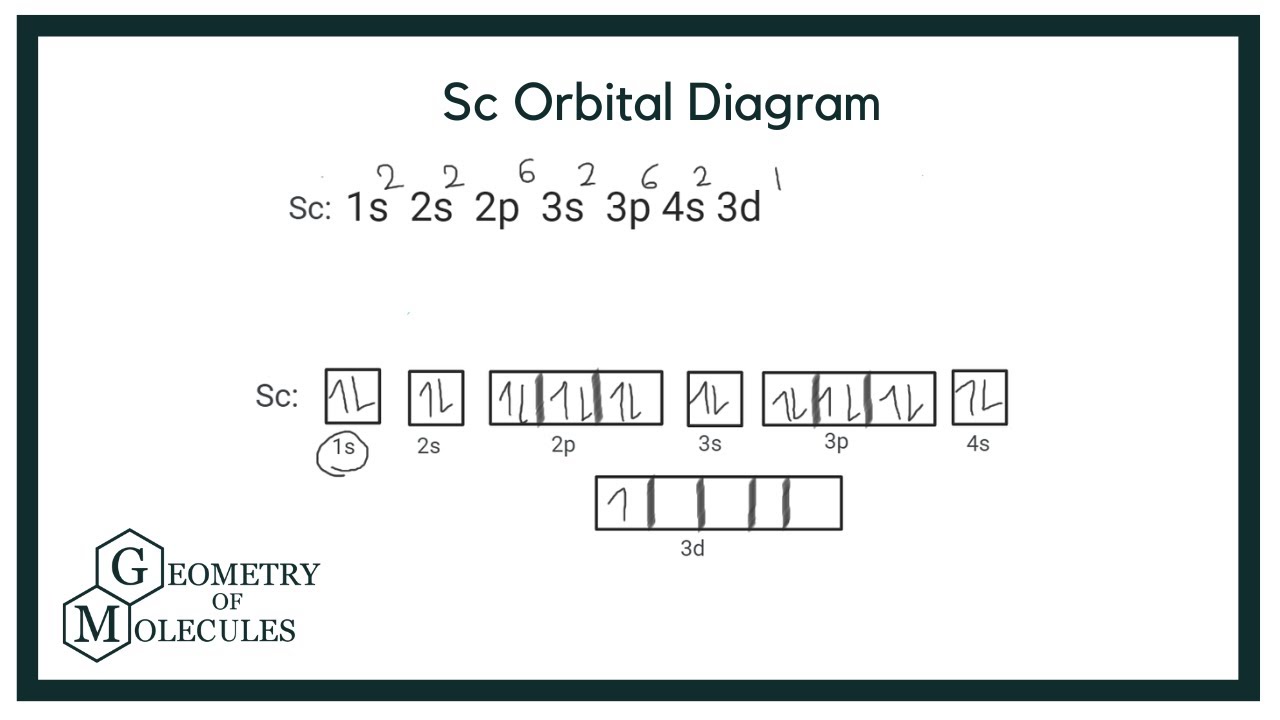
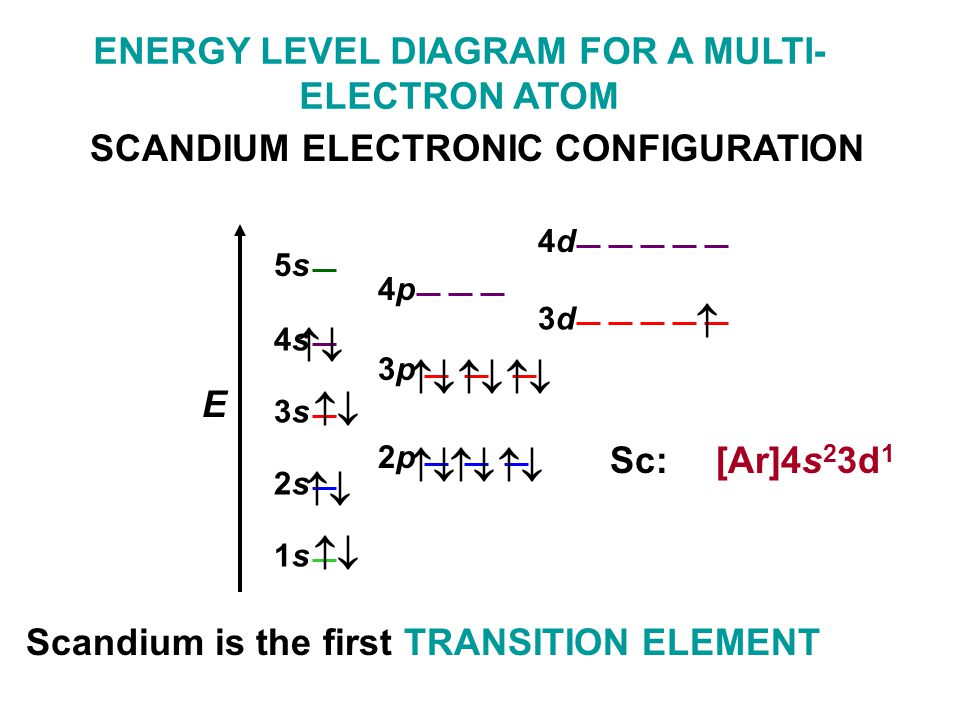
Draw The Electron Configuration For A Neutral Atom Of Scandium - Even though its electron configuration is a bit different, scandium still wants to bond with those three extra electrons, no matter what shell they are in. And so, it could have a similar electron configuration. 1s2 2s2 2p6 3s2 3p6 3d1 4s2. There are 2 steps to solve this one. There are 2 steps to solve this one. Distribution of electrons in shells is 2, 8, and 7. The electron filling order follows the sequence of 1s, 2s, 2p, 3s, 3p, 3d, and 4s orbitals. 3d14s2) but this really doesn't matter so much. Web scandium’s electron configuration is 1s² 2s² 2p6 3s² 3p6 3d¹ 4s². Web the electron configuration of a neutral atom of scandium (sc) can be determined by using the periodic table.
Electron configuration of sc is: The electron configuration for scandium can be determined by following the aufbau principle, which states that electrons fill orbitals from the lowest energy level to the highest. Well scandium has one more proton than calcium. Make sure to label each sublevel and populate with the appropriate numbers of electrons. More about the history and places to find scandium. In the diagram, the electrons are represented by small arrows that indicate their spin. 1s2 2s2 2p6 3s2 3p6 3d1 4s2. One of the significant uses of scandium in manufacturing industries is its inclusion in aluminum alloys. Locate the noble gas element in the period above the element of interest. Its atomic number is 21, which means it has 21 electrons.
You will find scandium to the right of calcium in the fourth period of the table. Write the electron configuration for a neutral atom of the element scandium. In order to write the sc electron configuration we first need to kn. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Draw the electron configuration for a neutral atom of scandium. The valence orbitals may have different ordering (i.e. This turns out to be argon 4s 1, 3d 1 or once again you could write argon, 3d 1, 4s 1. Draw the electron configuration for a neutral atom of boron. The electron configuration of scandium ion (sc 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6. There are 2 steps to solve this one.
Orbital Diagram For Scandium
Web the electron configuration of an atom describes how its electrons are arranged in different energy levels or shells. (b) how many valence electrons are found in this element? Well scandium has one more proton than calcium. Energy 3p click to change la. Electron configuration of scandium (sc) [ar] 3d 1 4s 2:
Orbital Diagram For Scandium
The electron configuration of an atom describes how its electrons are distributed among different energy levels and orbitals. Locate the noble gas element in the period above the element of interest. 3d14s2) but this really doesn't matter so much. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy.
Scandium Atom Science Notes and Projects
Make sure to label each sublevel and populate with the appropriate numbers of electrons. Web atomic number, atomic weight and charge of scandium ion. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 6.8.1 6.8. Write the electron configuration for a neutral atom of the element scandium..
How Can We Find Electron Configuration For Scandium Dynamic Periodic
1s 2 2s 2 2p 6. The electron configuration of scandium ion (sc 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6. O electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of scandium. Continue the electron configuration from the noble gas until you reach.
Atoms Diagrams Electron Configurations of Elements
Web this means that a neutral scandium atom will have 21 electrons. The electron configuration of an atom describes how its electrons are distributed among different energy levels and orbitals. You will find scandium to the right of calcium in the fourth period of the table. There are 2 steps to solve this one. This turns out to be argon.
Symbol and electron diagram for Scandium illustration Stock Vector
Draw the electron configuration for a neutral atom of boron. Continue the electron configuration from the noble gas until you reach the element of interest. Web atomic number, atomic weight and charge of scandium ion. Locate the atom on the periodic table. Web understanding the electron configuration of scandium.
Scandium Orbital Diagram
There are 2 steps to solve this one. In the diagram, the electrons are represented by small arrows that indicate their spin. Web scandium’s electron configuration is 1s² 2s² 2p6 3s² 3p6 3d¹ 4s². Web the electron configuration of an atom describes how its electrons are arranged in different energy levels or shells. A neutral helium atom, with an atomic.
Scandium Electron Configuration (Sc) with Orbital Diagram
More about the history and places to find scandium. Its atomic number is 21, which means it has 21 electrons. Web element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. (a) draw and orbital diagram for this element. Total number of protons in the nucleus is called the atomic number.
Draw Scandium's Electron Configuration
Web so now let's think about what the electron configuration of the scandium would be. Scandium donates the electron of the last shell to form bonds and turns into a scandium ion (sc 3+ ). Scandium is located in the fourth period, so it has four energy levels. O electronic structure drawing a box diagram of the electron configuration of.
3d render of atom structure of scandium isolated over white background
Draw the electron configuration for a neutral atom of iron. Locate the atom on the periodic table. Web so now let's think about what the electron configuration of the scandium would be. We place one electron in the orbital that is. Draw the electron configuration for a neutral atom of sodium.
Electron Configuration Of Titanium (Ti) [Ar] 3D 2 4S 2:
The electronic configuration is used to represent the el. Draw the electron configuration for a neutral atom of boron. 1s 2 2s 2 2p 6. Web the electron configuration of a neutral atom of scandium (sc) can be determined by using the periodic table.
Draw The Electron Configuration For A Neutral Atom Of Nickel.
Distribution of electrons in shells is 2, 8, and 7. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Sc, z=21 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^1 we account for 21 electrons. Web this means that a neutral scandium atom will have 21 electrons.
Web Scandium’s Electron Configuration Is 1S² 2S² 2P6 3S² 3P6 3D¹ 4S².
The electron configuration of scandium can be written as 1s22s22p63s23p64s23d1. Web the electron configuration of an atom describes how its electrons are arranged in different energy levels or shells. Scandium donates the electron of the last shell to form bonds and turns into a scandium ion (sc 3+ ). Well scandium has one more proton than calcium.
The Electron Configuration Of Scandium Ion (Sc 3+) Is 1S 2 2S 2 2P 6 3S 2 3P 6.
Draw the electron configuration for a neutral atom of sodium. Electron configuration of scandium (sc) [ar] 3d 1 4s 2: That is, scandium is a cation element. Web for example if you form the scandium plus one ion, the electron configuration for the scandium plus one ion, so we're losing an electron from a neutral scandium atom.



:max_bytes(150000):strip_icc()/Scandium-58b6023e3df78cdcd83d49e1.jpg)