Draw The Lewis Structure For A Oxygen Molecule
Draw The Lewis Structure For A Oxygen Molecule - Find more chemistry widgets in wolfram|alpha. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. What is an example of a lewis structures practice problem? Draw lewis structures depicting the bonding in simple molecules. By the end of this section, you will be able to: Distribute the remaining electrons as lone pairs on the terminal atoms. 1st attempt part 1 (1 point) draw the lewis structure for o2. Web 22k views 3 years ago. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond.
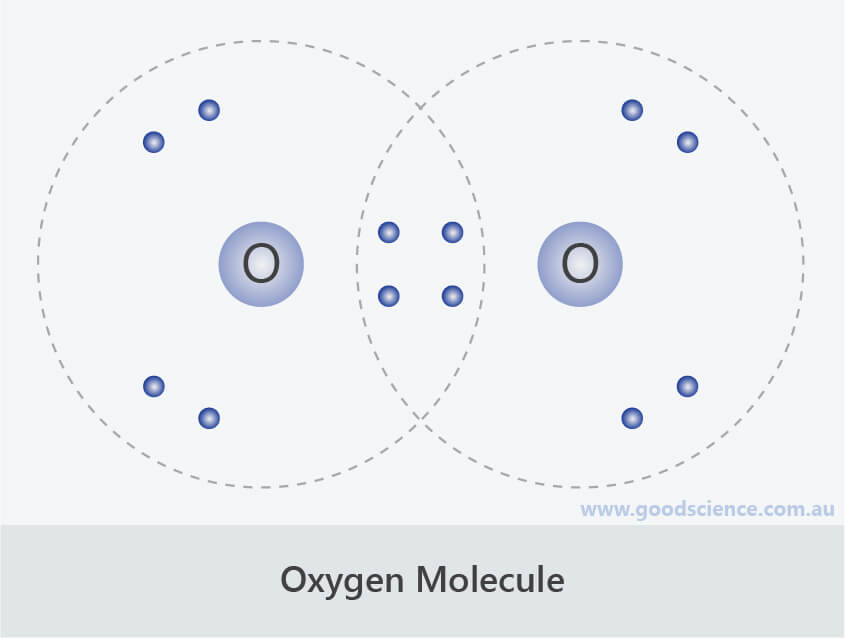
In this case, there is no central atom, so we distribute the electrons around both atoms. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. There are 12 valence electrons available for the lewis structure for o 2. Distribute the remaining electrons as lone pairs on the terminal atoms. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. 6.1 lewis symbols and structures. Find total number of electrons of the valance shells of oxygen atoms. The only thing smaller than atoms is their subatomic particles; Oxygen lewis dot structures with itself and other elements can be used for determining chemical bond formation. Draw lewis structures depicting the bonding in simple molecules.
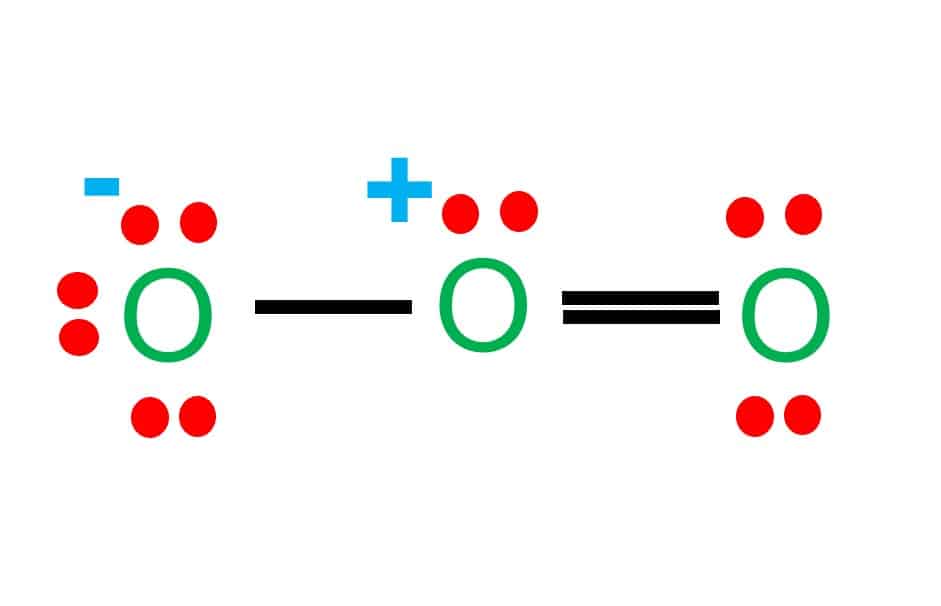
Two fluorine atoms can form a molecule of f 2 in the same fashion. The only thing smaller than atoms is their subatomic particles; Web draw a skeleton structure of the molecule. Web in this case, the lewis structure is inadequate to depict the fact that experimental studies have shown two unpaired electrons in each oxygen molecule. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Web using lewis structures, we can represent this as follows: 1st attempt part 1 (1 point) draw the lewis structure for o2. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Note that diatomic oxygen is often called molecular oxygen or just.
【2 Step】O2 Lewis StructureLewis Dot Structure for Oxygen(O,O2)Lewis
Atoms can form more than one bond. Determine total electrons pairs existing as lone pairs and bonds. What is an example of a lewis structures practice problem? Be sure to draw all bonds and lone pairs. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle.
O3 Lewis Structure Step By Step Drawing What's Insight
This widget gets the lewis structure of chemical compounds. Draw lewis structures depicting the bonding in simple molecules. In order to draw the lewis structure of o2, first of all you have to find the total number of valence electrons present in the o2 molecule. Find more chemistry widgets in wolfram|alpha. Send feedback | visit wolfram|alpha.
O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Draw lewis structures depicting the bonding in simple molecules. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond..
O2 2 Lewis Structure How to Draw the Lewis Structure for O2 2 YouTube
In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Web in this case, the lewis structure is inadequate to depict the fact that experimental studies have shown two unpaired electrons in each oxygen molecule. 6.1 lewis symbols and structures. The atomic number of oxygen is 8 and its electronic configuration is 2,6. (note that.
Lewis Structure Of Oxygen
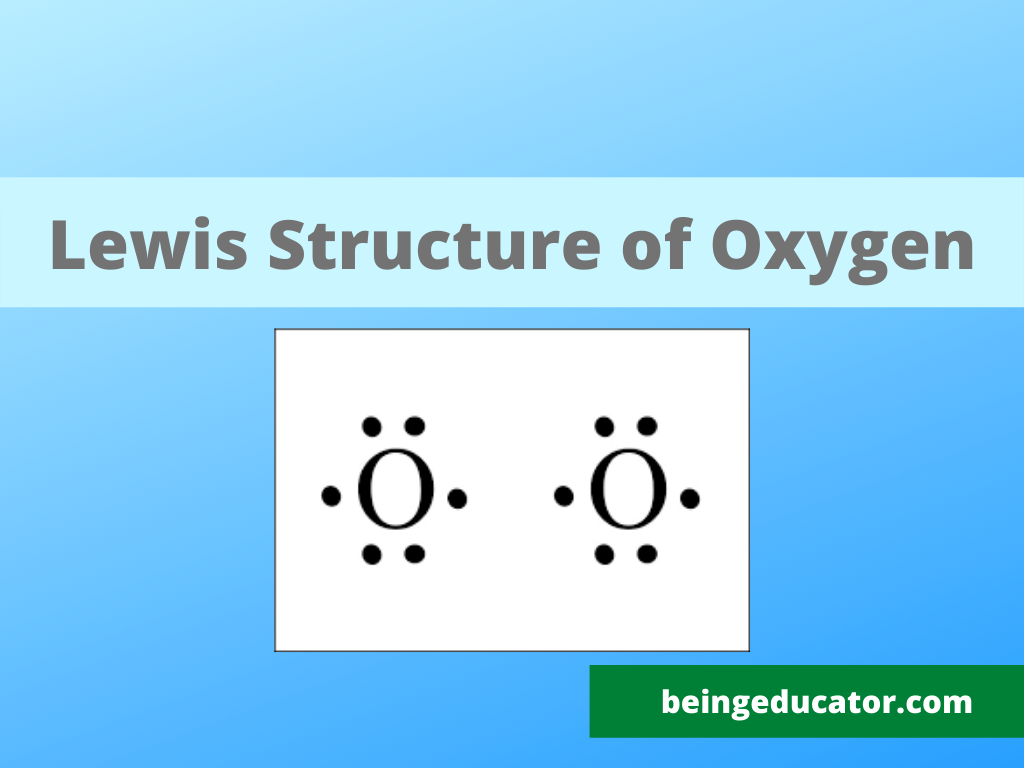
Web lewis structure of o2. 6.1 lewis symbols and structures. Draw lewis structures for molecules. Write lewis symbols for neutral atoms and ions. 318k views 11 years ago.
O2 Lewis Structure
In this case, there is no central atom, so we distribute the electrons around both atoms. Web oxygen (o 2) is a commonly tested lewis structure due to it's importance on earth. Web added jun 9, 2014 by webtester in chemistry. Web lewis structure of o2. Web draw a skeleton structure of the molecule.
rules for drawing lewis dot structures for molecules
Web because o 2 is a simple molecule, it is extremely easy to draw the lewis structure of it. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. By the end of this section, you will be able to: This widget gets the lewis structure of chemical compounds. In a.
Electron Dot Structure For Oxygen
Send feedback | visit wolfram|alpha. Write lewis symbols for neutral atoms and ions. 6 steps to draw the lewis structure of o2. Note that each atom must contribute one electron to the bond. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom.
How to Draw a Lewis Structure
This widget gets the lewis structure of chemical compounds. Note that diatomic oxygen is often called molecular oxygen or just. 1st attempt part 1 (1 point) draw the lewis structure for o2. What is an example of a lewis structures practice problem? Find more chemistry widgets in wolfram|alpha.
O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Send feedback | visit wolfram|alpha. Draw lewis structures for molecules. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Find total number of electrons of the valance shells of oxygen atoms. For very simple molecules and molecular.
Web Using Lewis Structures, We Can Represent This As Follows:
Atoms can form more than one bond. Distribute the remaining electrons as lone pairs on the terminal atoms. 1st attempt part 1 (1 point) draw the lewis structure for o2. (note that we denote ions with brackets around the.
6 Steps To Draw The Lewis Structure Of O2.
By the end of this section, you will be able to: The atomic number of oxygen is 8 and its electronic configuration is 2,6. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Be sure to draw all bonds and lone pairs.
By The End Of This Section, You Will Be Able To:
The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Write lewis symbols for neutral atoms and ions. 318k views 11 years ago. Web oxygen (o 2) is a commonly tested lewis structure due to it's importance on earth.
Practice With Drawing Lewis Structures.
Find total number of electrons of the valance shells of oxygen atoms. Drawing the lewis structure for. Two fluorine atoms can form a molecule of f 2 in the same fashion. Note that diatomic oxygen is often called molecular oxygen or just.
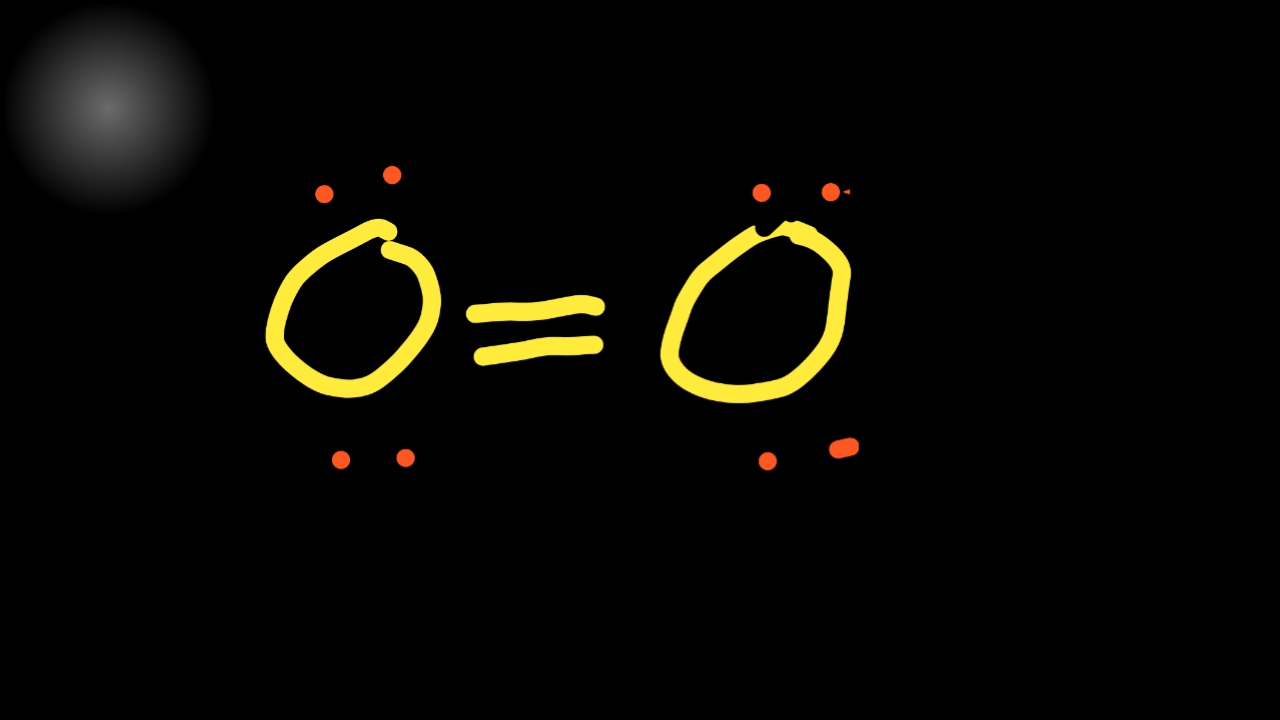



:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)

