Draw The Lewis Structure For Cs2
Draw The Lewis Structure For Cs2 - #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure. Calculate the number of valence electrons: Web use these steps to correctly draw the cs 2 lewis structure: Web this widget gets the lewis structure of chemical compounds. After determining how many valence electrons there are in cs2, place them around the central atom to complete the octets. Web steps of drawing cs2 lewis structure. Web to draw the cs2 lewis structure, one must first determine the number of valence electrons for each atom, which is 4 for carbon and 6 for sulfur. Connect the atoms with dots: Web drawing the lewis structure for cs 2. Web to draw the lewis structure for cs2, follow these steps:
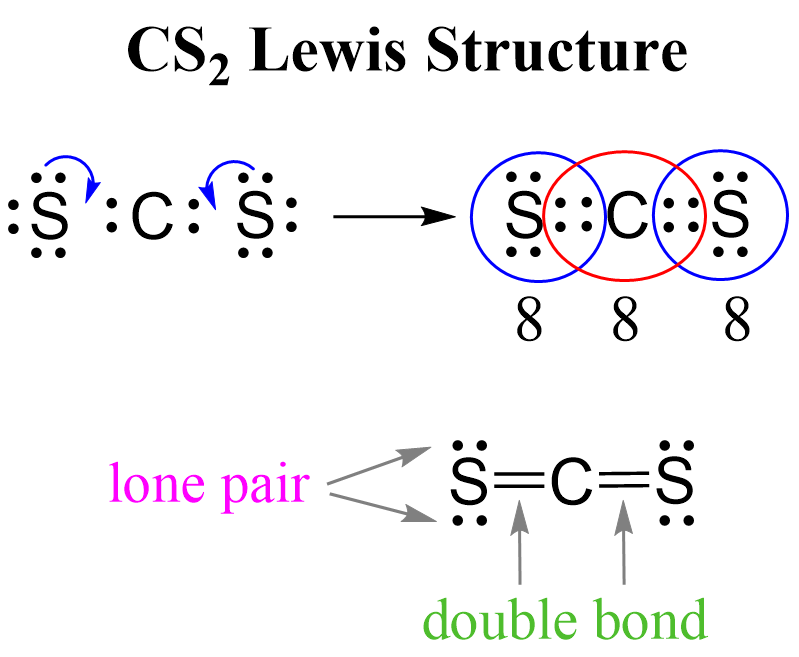
Web to draw the cs2 lewis structure, one must first determine the number of valence electrons for each atom, which is 4 for carbon and 6 for sulfur. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Web in short, these are the steps you need to follow for drawing a lewis structure: Distribution of remaining valence electrons: We draw lewis structures to predict: There are three single bonds between the carbon atom (c) and the sulfur atoms (s). 6.9k views 1 year ago. #3 calculate and mark formal charges on the atoms, if required. Sum the valence electrons from all the atoms. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle.
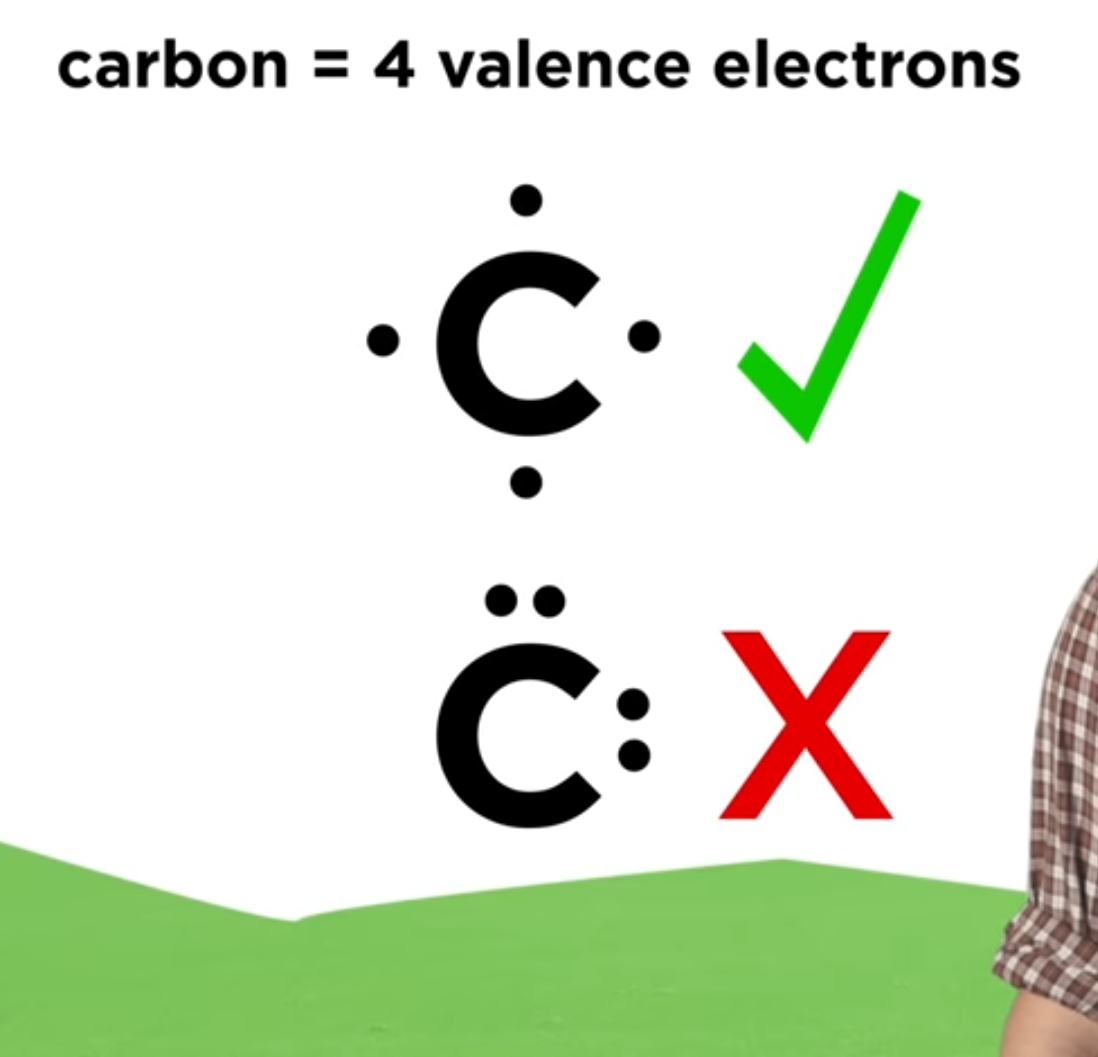
#5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure. In this case, we can condense the last few steps, since not all of them apply. Draw a lewis structure for cs2 draw the molecule by placing atoms on the grid and connecting them with bonds. #1 first draw a rough sketch. 8 + (6 × × 7) = 50; Web this is how we draw a complete lewis structure of carbon disulfide. Web in short, these are the steps you need to follow for drawing a lewis structure: * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. For the cs2 lewis structure, calculate the total number of valence electrons for the cs2 molecule. For the cs2 structure use the periodic table to find the total.
CS2 Lewis Structure in 4 simple Steps with images What's Insight
Carbon (c) possesses 4 valence electrons, while sulfur (s) has 6 valence electrons. For the cs2 structure use the periodic table to find the total. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Calculate the total number of valence electrons in cs2: When drawing the structure.
CS2 Lewis Structure How to Draw the Lewis Structure for CS2 YouTube
8 + (6 × × 7) = 50; In order to find the total valence electrons in cs2 (carbon disulfide) molecule, first of all you should know the valence electrons present in carbon atom as well as sulfur atom. Then, the electrons are placed around the atoms to satisfy the octet rule, which states that each atom should have eight.
CS2 Lewis Structure, Geometry, and Hybridization Chemistry Steps
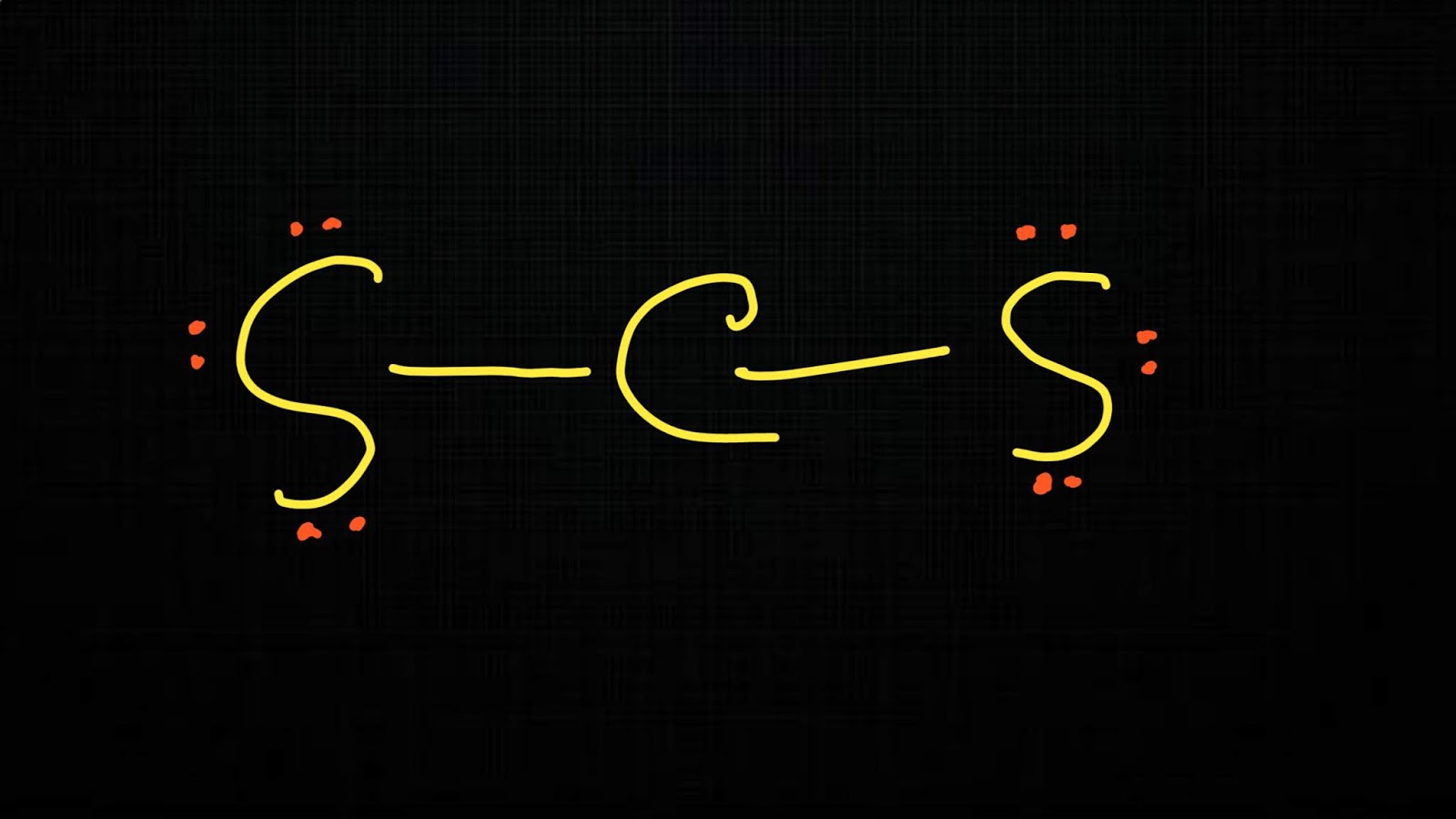
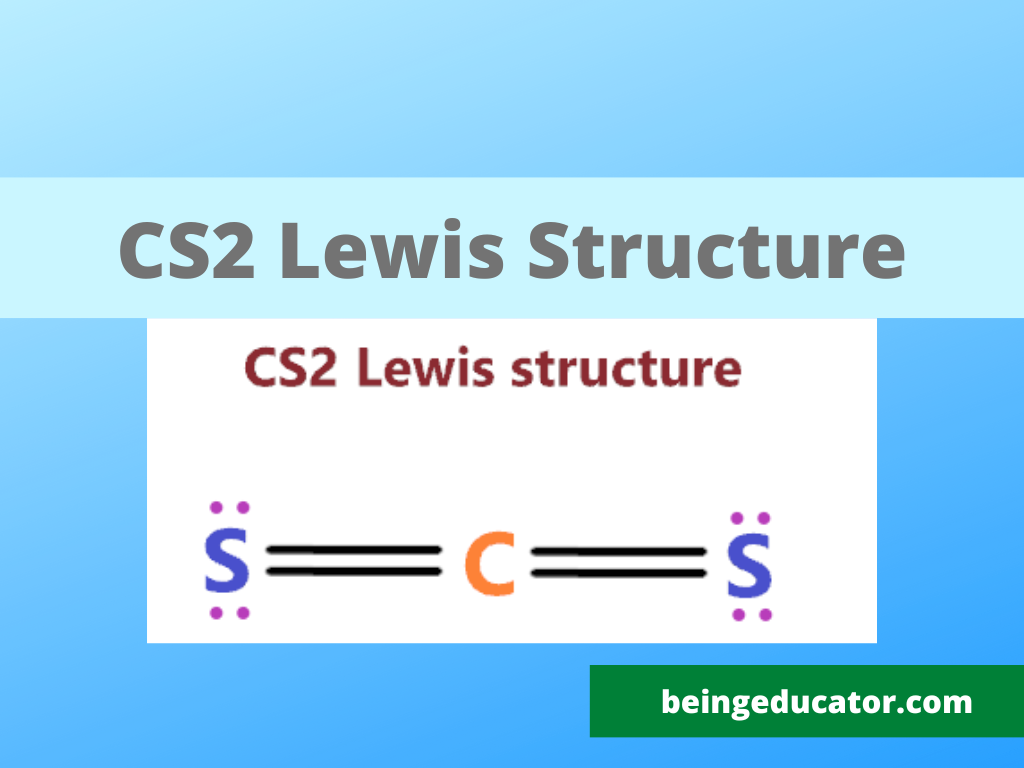
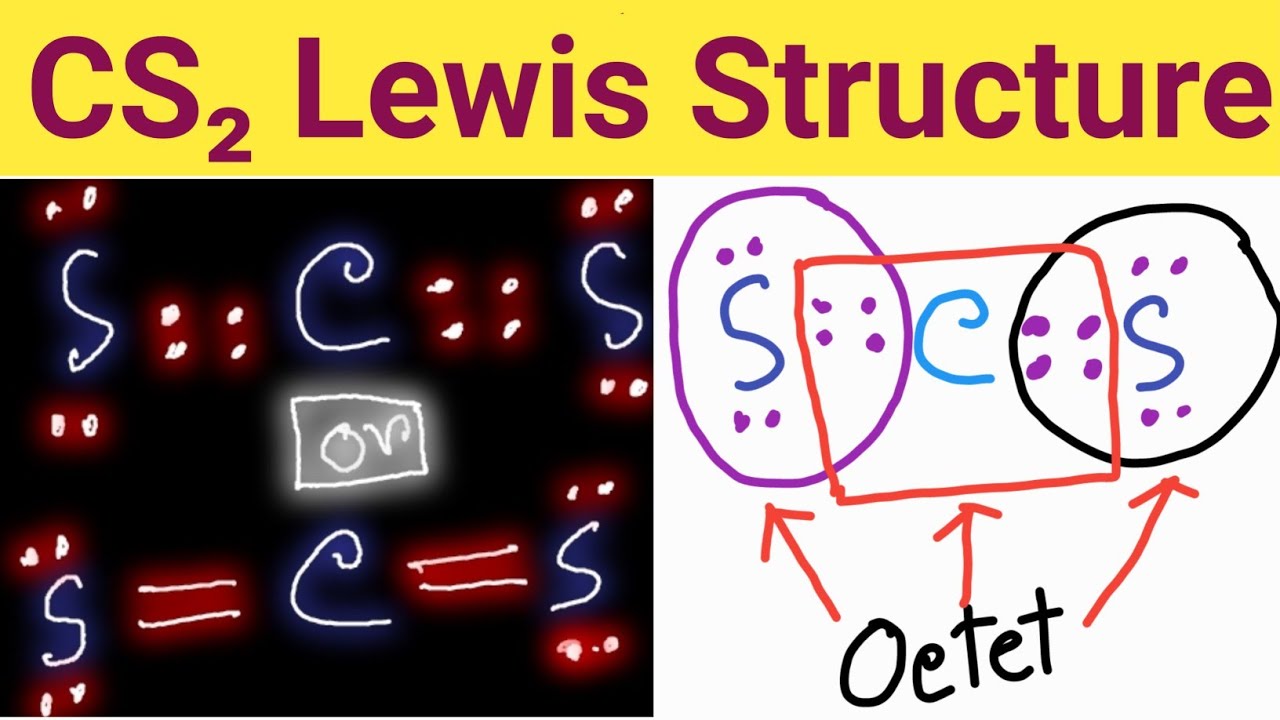
Include all lone pairs of electrons. Both sulfur atoms have a full octet, and carbon has a formal charge of zero. Check if all atoms have an octet: Lewis structure is the structural representation of the number of valence electrons that participate in the bond formation and nonbonding electron pairs. Draw the molecule by placing atoms on the grid and.
How do you draw the Lewis structure of CS2 (Carbon disulfide) YouTube
Draw a skeleton joining the atoms by single bonds. Check if all atoms have an octet: After determining how many valence electrons there are in cs2, place them around the central atom to complete the octets. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. * hydrogen atoms are always terminal.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
In order to find the total valence electrons in cs2 (carbon disulfide) molecule, first of all you should know the valence electrons present in carbon atom as well as sulfur atom. Web use these steps to correctly draw the cs 2 lewis structure: Lewis structure is the structural representation of the number of valence electrons that participate in the bond.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
There are three single bonds between the carbon atom (c) and the sulfur atoms (s). Web steps of drawing cs2 lewis structure. Web to draw the cs2 lewis structure, one must first determine the number of valence electrons for each atom, which is 4 for carbon and 6 for sulfur. We draw lewis structures to predict: Both sulfur atoms have.
Lewis Dot Structure for CS2 Carbon disulfide YouTube
* hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. In order to draw the lewis structure of cs2, first of all you have to find the total number of valence electrons present in the cs2 molecule. #2 mark lone pairs on the atoms. Include all lone pairs of electrons. Web drawing the.
CS2 Lewis Structure Molecular Geometry Polarity Hybridization
In order to find the total valence electrons in cs2 (carbon disulfide) molecule, first of all you should know the valence electrons present in carbon atom as well as sulfur atom. Web steps of drawing cs2 lewis structure. Web the correctly drawn lewis structure for cs2 has no nonbonding electrons. As there are two sulfur atoms, the total valence electrons.
CS2 Lewis Structure Lewis Dot Structure for CS2 Carbon Disulfide
8 + (6 × × 7) = 50; Draw the lewis electron dot structures for these molecules,. 6.9k views 1 year ago. Draw a lewis structure for nci: For the cs2 structure use the periodic table to find the total.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Web in short, these are the steps you need to follow for drawing a lewis structure: Then, the electrons are placed around the atoms to satisfy the octet rule, which states that each atom should have eight electrons in its valence shell. Determine the central metal atom: Here, the given molecule is cs2 (carbon disulfide). Do you know that atoms.
6.9K Views 1 Year Ago.
24k views 11 years ago chemistry lewis dot structures. Calculate the total number of valence electrons in cs2: Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Then, the electrons are placed around the atoms to satisfy the octet rule, which states that each atom should have eight electrons in its valence shell.
There Are Three Single Bonds Between The Carbon Atom (C) And The Sulfur Atoms (S).
Distribution of remaining valence electrons: So, option b is incorrect. Web to draw the lewis structure for cs2, follow these steps: Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom.
Connect The Atoms With Dots:
Web to draw the cs2 lewis structure, one must first determine the number of valence electrons for each atom, which is 4 for carbon and 6 for sulfur. Web in short, these are the steps you need to follow for drawing a lewis structure: Draw a lewis structure for nci: #3 calculate and mark formal charges on the atoms, if required.
Draw A Lewis Structure For Cs2 Draw The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
In order to find the total valence electrons in cs2 (carbon disulfide) molecule, first of all you should know the valence electrons present in carbon atom as well as sulfur atom. #1 first draw a rough sketch. Include all lone pairs of electrons. In this case, we can condense the last few steps, since not all of them apply.