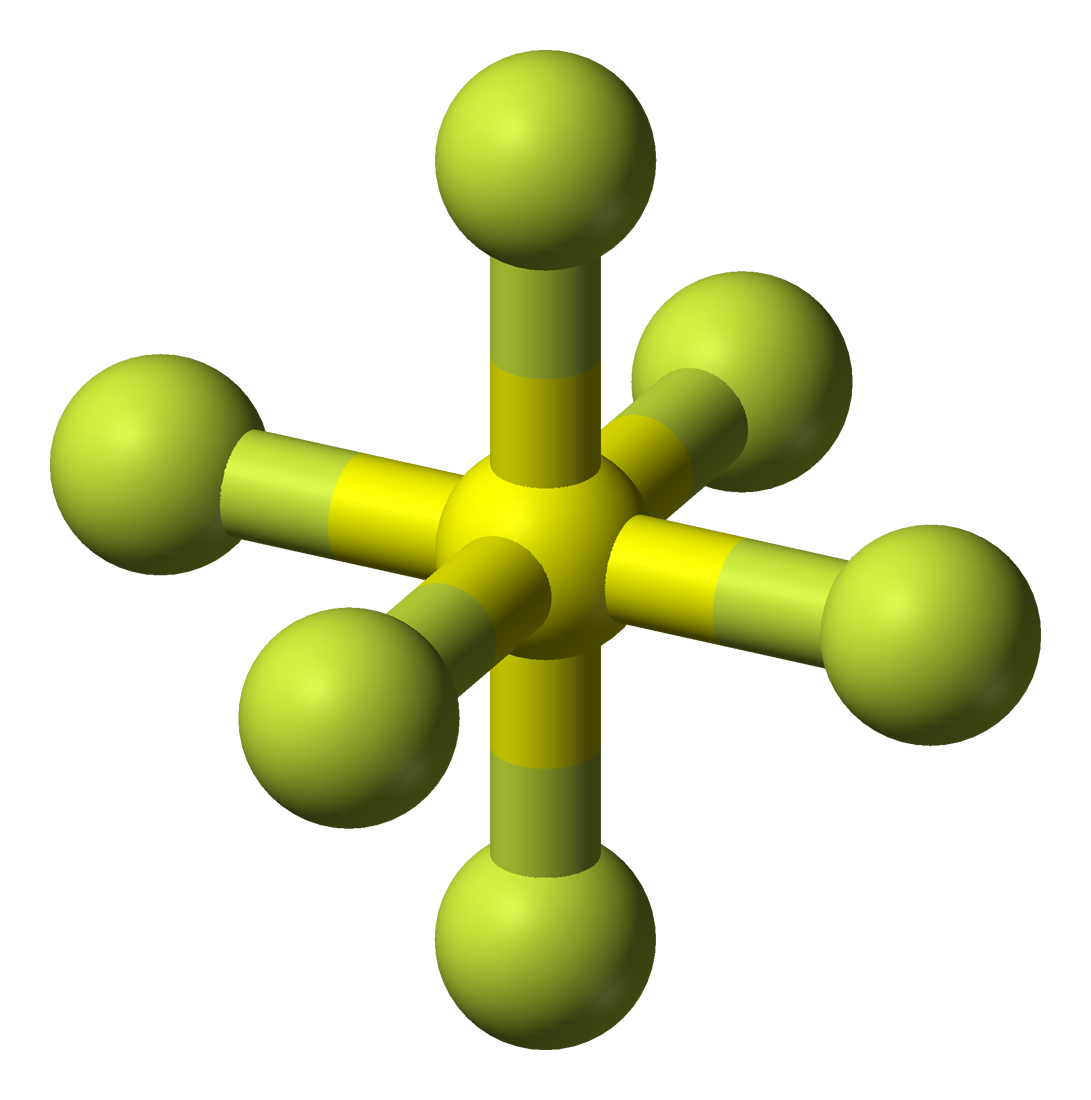
Draw The Lewis Structure For Sf6
Draw The Lewis Structure For Sf6 - 161k views 10 years ago. The central atom here is sulfur bonded with 6 fluorine atoms. 69k views 12 years ago every video. Find more chemistry widgets in wolfram|alpha. There’s just one step to solve this. 100% (3 ratings) step 1. In this tutorial, we will learn how to draw the lewis structure of sf 4 step by step by covering all theories. In order to draw the lewis structure of sf6, first of all you have to find the total number of valence electrons present in the sf6 molecule. #2 mention lone pairs on the atoms. Web in this article, “sf6 lewis structure”, hybridization, geometry, formal charge along with some detailed explanations are discussed briefly.
#2 mention lone pairs on the atoms. 161k views 10 years ago. #3 if needed, mention formal charges on the atoms. Which of the following statements are correct. Draw lewis dot (electron) structure for sf6 and determine the following: C) molecular geometry of sf6. By using the following steps, you can easily draw the lewis structure of sf 6: 4k views 11 years ago chemistry lewis dot structures. A) electron geometry b) molecular geometry c) central atom hybridization d) polar or nonpolar. The central atom here is sulfur bonded with 6 fluorine atoms.
Web in this article, “sf6 lewis structure”, hybridization, geometry, formal charge along with some detailed explanations are discussed briefly. #3 if needed, mention formal charges on the atoms. The lewis dot structure of any molecule is a pictorial representation of the atoms involved in forming the structure and its individual valence electrons. A) electron geometry b) molecular geometry c) central atom hybridization d) polar or nonpolar. (valence electrons are the number of electrons present in the outermost shell of an atom). Web draw a lewis structure for sf6 that has minimized formal changes. We’ll look at each stage of drawing the lewis structure of sulfur hexafluoride (sf6). Web expanded octet lewis structure: 4k views 11 years ago chemistry lewis dot structures. Web lewis structure for sf6.
Drawn Molecule Sf6 Sf6 Lewis Dot Structure Free Transparent PNG
(valence electrons are the number of electrons present in the outermost shell of an atom). There are 2 steps to solve this one. Include all nonbonding electrons and any nonzero formal charges. Which of the following statements are correct. Here, the given molecule is sf6 (sulfur hexafluoride).
La geometría molecular SF6, la Estructura, la Forma y la polaridad de
Web draw the lewis structure for sf6, and then answer the following questions: Which of the following statements are correct. This problem has been solved! Sf 6 is a lewis structure with sulfur (s) which can hold more than 8 valence electrons. This widget gets the lewis structure of chemical compounds.
Sf6 lewis structure
The shape is square pyramidal. 161k views 10 years ago. A video explanation of how to draw the lewis dot structure for sulfur hexafluoride,. Find the total valence electrons in sf6 molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
LEWIS STRUCTURE OF SF6
A video explanation of how to draw the lewis dot structure for sulfur hexafluoride,. Web draw the lewis structure for sf6, and then answer the following questions: In this tutorial, we will learn how to draw the lewis structure of sf 4 step by step by covering all theories. Lewis dot structure has 6 sigma bonds and rests lone pairs.
Sf6 lewis structure
The lewis structure of a molecule represents the distribution of electrons in the molecule. Web drawing the lewis structure for sf 6. B) electron domain geometry of sf6. In order to draw the lewis structure of sf6, first of all you have to find the total number of valence electrons present in the sf6 molecule. The steric number is 6.
SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
The steric number is 4 the steric number is 5. Sf 6 is a lewis structure with sulfur (s) which can hold more than 8 valence electrons. The steric number is 6. Since there are seven fluorine (f) atoms it will be necessary. 100% (3 ratings) step 1.
Sf6 lewis structure
Web lewis structure for sf6. There are 48 valence electrons. #3 if needed, mention formal charges on the atoms. We’ll look at each stage of drawing the lewis structure of sulfur hexafluoride (sf6). C) molecular geometry of sf6.
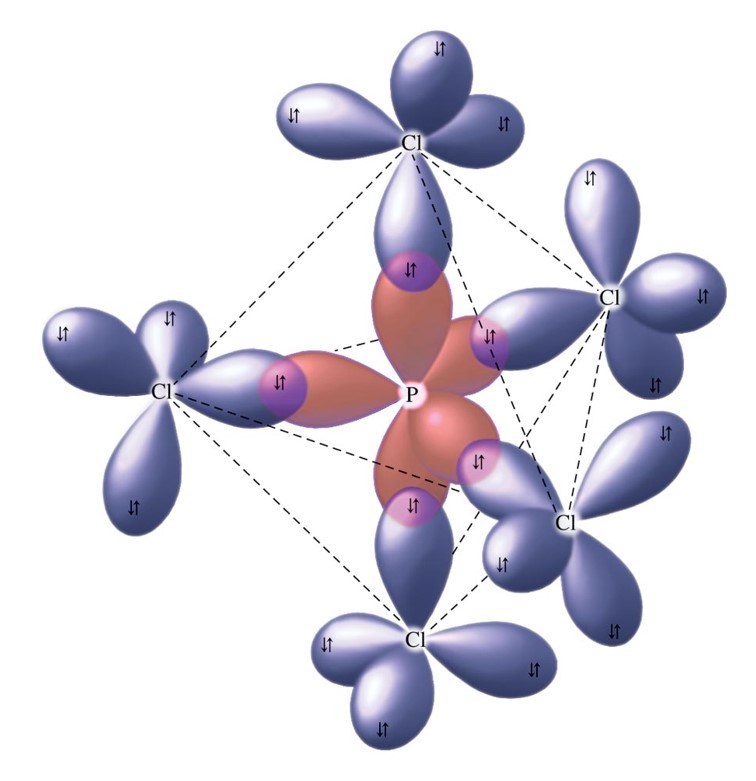
Molecular Geometry of SF6 [with video and free study guide]
Web draw the lewis structure for sf6, and then answer the following questions: Calculate the total number of valence electrons. In this tutorial, we will learn how to draw the lewis structure of sf 4 step by step by covering all theories. How to draw the lewis structure of sf6. Sulfur can have an expanded octet, which means it is.
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity
I also go over the hybridization, shape and bond angles. The central atom here is sulfur bonded with 6 fluorine atoms. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find the total valence electrons in sf6 molecule. Calculate the total number of valence electrons.
Find More Chemistry Widgets In Wolfram|Alpha.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web in the sf 6 lewis structure, there are six single bonds around the sulfur atom, with six fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. 4k views 11 years ago chemistry lewis dot structures. Draw the lewis structure and shape for sf6.
Which Of The Following Statements Are Correct.
The central atom here is sulfur bonded with 6 fluorine atoms. There are 2 steps to solve this one. Web in sf 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. We’ll look at each stage of drawing the lewis structure of sulfur hexafluoride (sf6).
2.1K Views 10 Months Ago.
Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. Web draw a lewis structure for sf6 that has minimized formal changes. #1 draw a rough skeleton structure. C) molecular geometry of sf6.
Draw Lewis Dot (Electron) Structure For Sf6 And Determine The Following:
By using the following steps, you can easily draw the lewis structure of sf 6: Here, the given molecule is sf6 (sulfur hexafluoride). #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom.
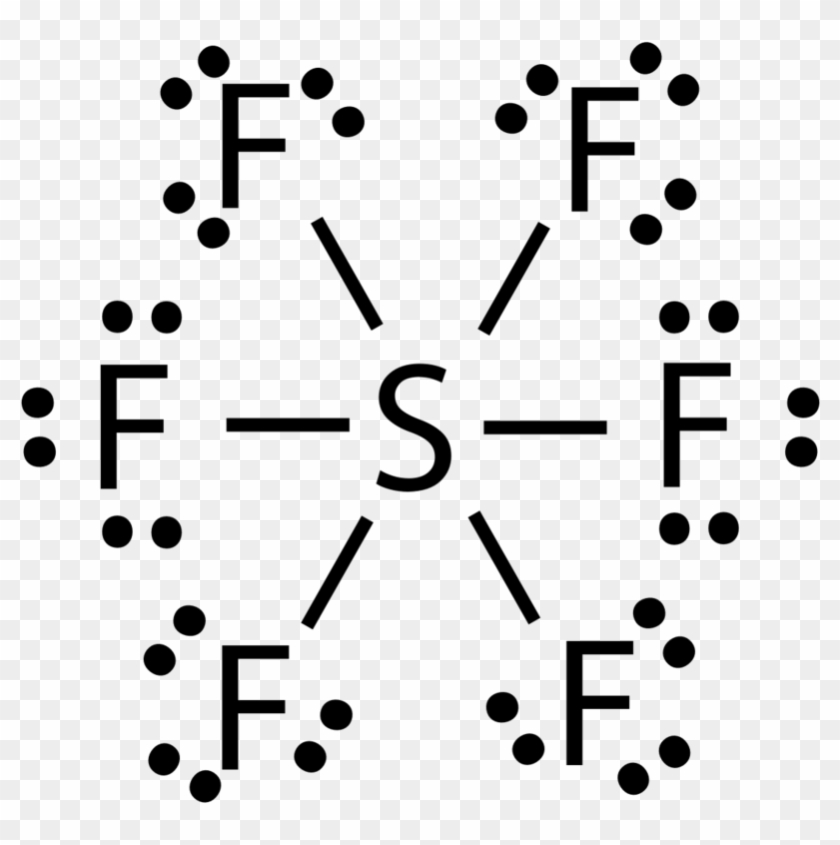
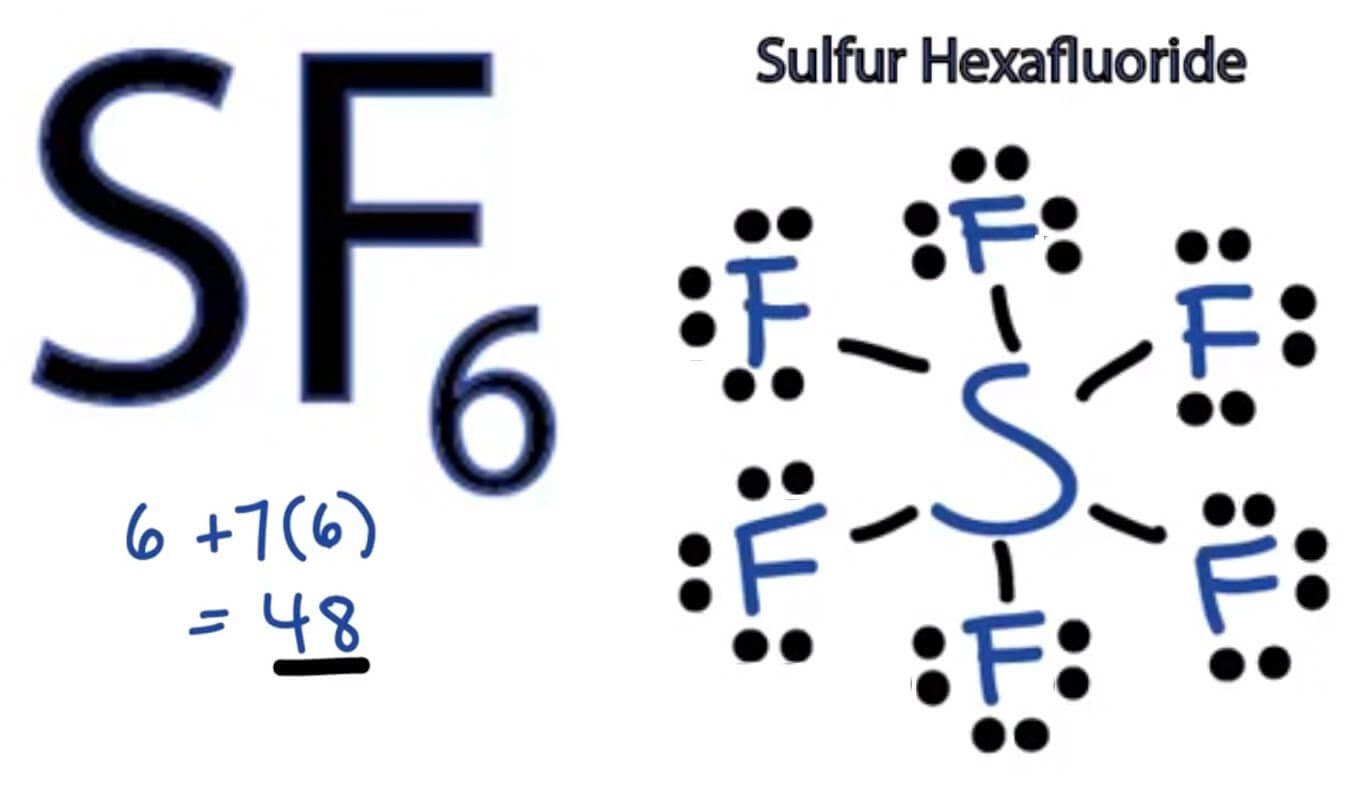


![Molecular Geometry of SF6 [with video and free study guide]](https://aceorganicchem.com/chemistry/wp-content/uploads/2023/06/SF6-lewis-structures-1024x584.jpg)