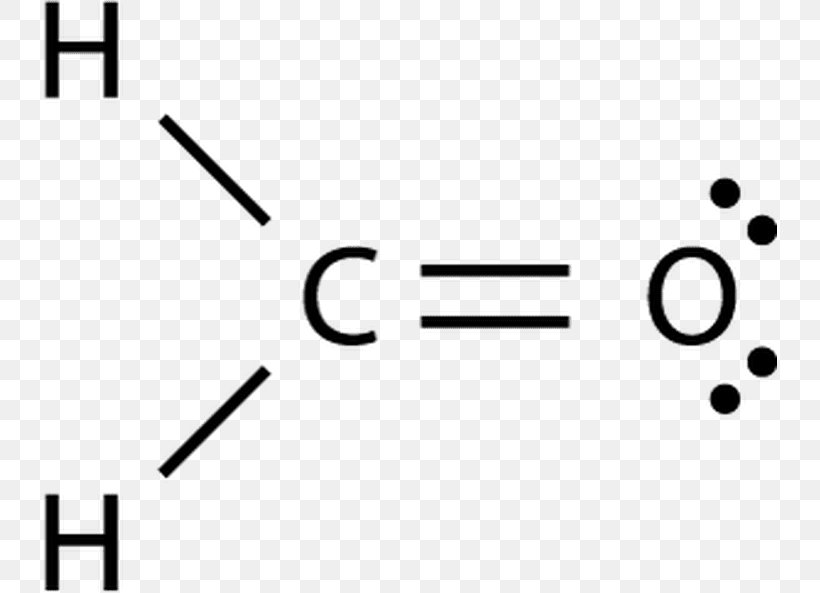
Draw The Lewis Structure For The Formaldehyde
Draw The Lewis Structure For The Formaldehyde - There is a double bond between oxygen and carbon atom. There are 12 valence atoms total. Search for how many more electrons are required to stabilize the octet. Web here’s the best way to solve it. Eight of these electrons are tied up in bonds. Place electrons around outside atoms. Draw the lewis structure of formaldehyde (h2co). Formaldehyde (hcho) contains two hydrogen atoms, one carbon atom and one oxygen atom. For further queries comment down below. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4.
Web formaldehyde lewis structure. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. 100% (18 ratings) hope it'll be helpful. Just click or tap on the plus symbol to open the drawing tool. Thus it is also called dot and line structure. The last step of drawing the lewis structure of the compound is to connect the atoms with bonds. Place electrons around outside atoms. Next draw out the framework of the molecule: Formaldehyde is a highly reactive molecule and is known to cause irritation and other health effects when inhaled. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts.
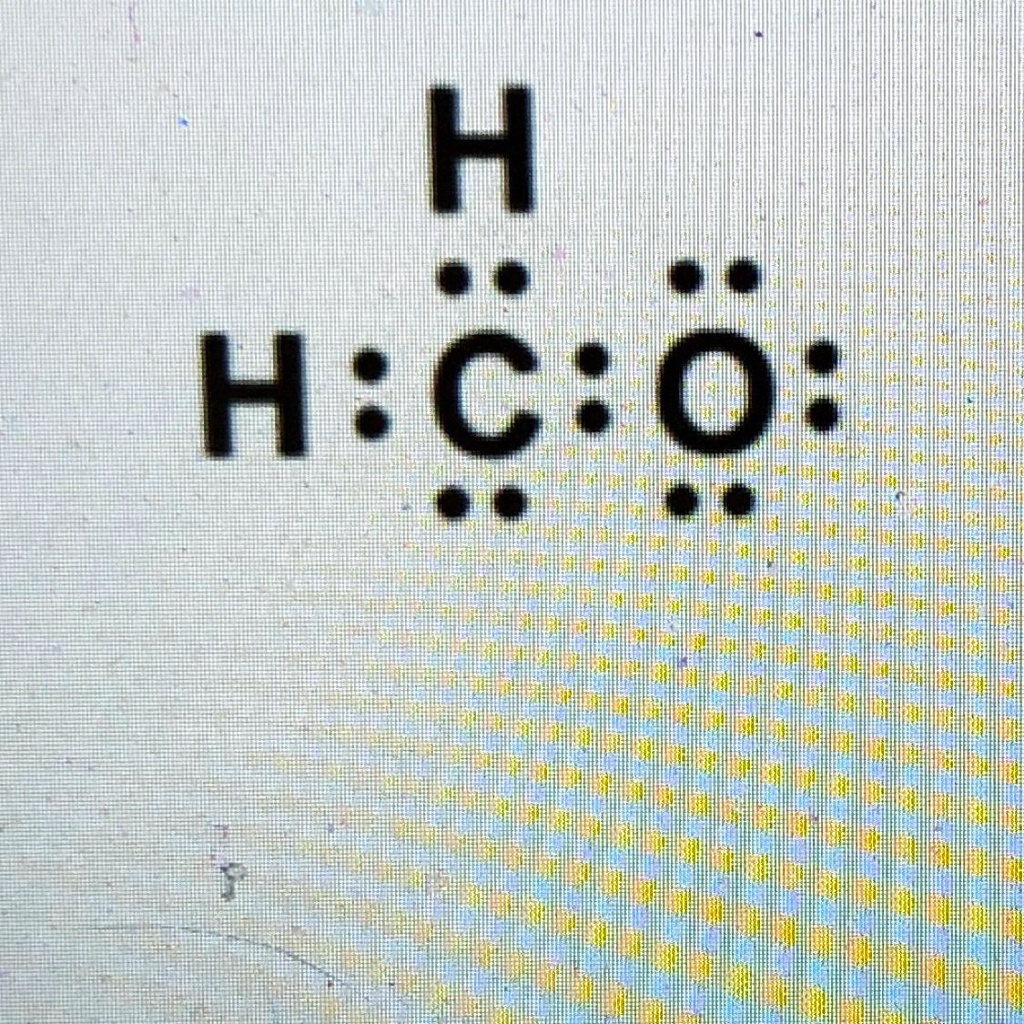
Our example is a question on drawing the structure of formaldehyde or h 2 co. Formaldehyde is a highly reactive molecule and is known to cause irritation and other health effects when inhaled. 4 + (3 × 6) + 2 = 24 electrons. Molecular geometry of ch 2 o. Web formaldehyde (hcho, ch 2 o, methanal) molecule lewis structure. Each atom in the molecule has a complete outer shell full of electrons. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of a molecule can be constructed using the following stepwise procedure: The remaining four complete the octet around the oxygen atom. Constructing the lewis structure of the formaldehyde (h 2 co) molecule. When the question is first opened, a box with a plus symbol is shown below the question.
Draw a Lewis Structure of Formaldehyde in 2020 Molecular geometry
One line is a single bond with 2 bonding electrons, two lines is a double bond with 4. There are no electrons left over and the structure is complete. *carbon can form a maximum of 4 bonds by sharing its four va. Web lewis structure is a simplified diagram which shows the bonding of atoms in a molecule. Lewis diagram.
Formaldehyde Structure, Formula, Effects, Uses
It is a colorless gas with a pungent odor. Web formaldehyde (hcho, ch 2 o, methanal) molecule lewis structure. Lewis diagram of formaldehyde (ch₂o) the lewis diagram of a molecule can be constructed using the following stepwise procedure: *carbon can form a maximum of 4 bonds by sharing its four va. Draw the lewis structure for the formaldehyde (ch2o) molecule.
SOLVED 'Examine the Lewis structure below. If it is a correct
Web formaldehyde lewis structure. Draw the lewis structure for the formaldehyde (ch2o) molecule. There are 12 valence atoms total. 100% (18 ratings) hope it'll be helpful. Thus it is also called dot and line structure.
Formaldehyde Molecular Geometry
Web this problem has been solved! Next draw out the framework of the molecule: Draw the lewis structure for the formaldehyde (ch2o) molecule. The last step of drawing the lewis structure of the compound is to connect the atoms with bonds. Constructing the lewis structure of the formaldehyde (h 2 co) molecule.
What is the Lewis structure for formaldehyde? YouTube
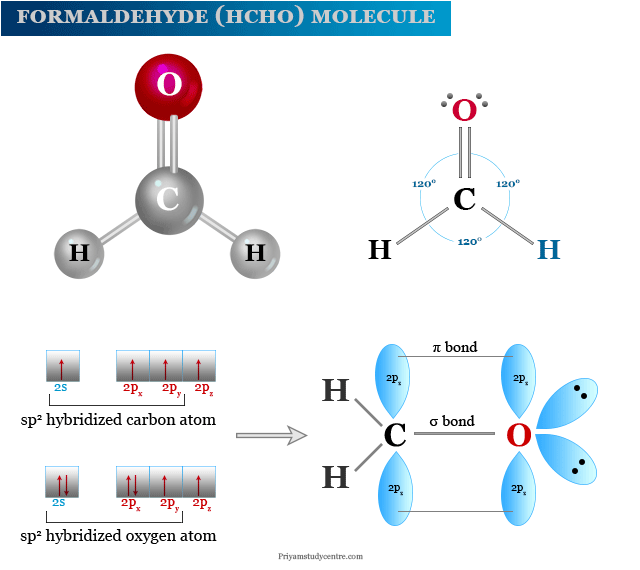
Web on the other hand, the amounts of electronegativity of oxygen and carbon atoms are 3.44 and 2.55 respectively.as carbon is the least electronegative atom in the group of the atoms in formaldehyde, it takes the central position. Next draw out the framework of the molecule: Search for how many more electrons are required to stabilize the octet. There are.
How to Draw the Lewis Dot Structure for CH2O (Formaldehyde) YouTube
Each atom in the molecule has a complete outer shell full of electrons. Carbon atom is located as the center atom in hcho. Did you know that formaldehyde is a naturally occurring compound? Search for how many more electrons are required to stabilize the octet. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”).
How to Draw a Lewis Structure Lewis, Chemistry, Draw
100% (18 ratings) hope it'll be helpful. Yes, covalent bonds come in pairs which are represented by lines in lewis structures. When the question is first opened, a box with a plus symbol is shown below the question. Thus it is also called dot and line structure. View the full answer step 2.
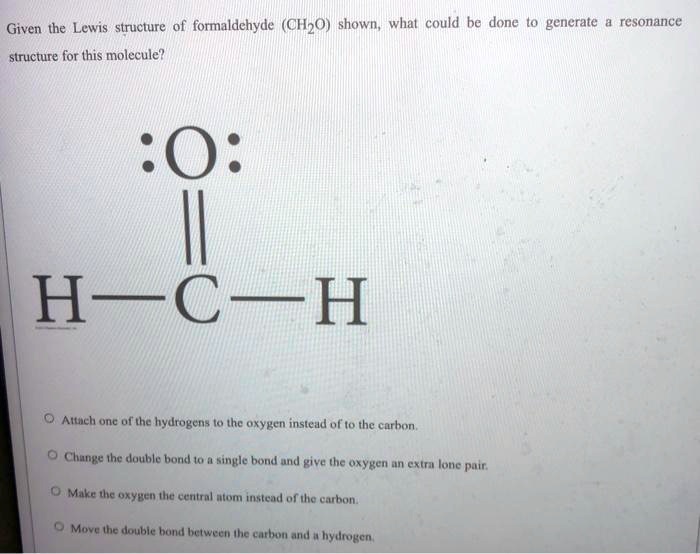
SOLVED Given the Lewis structure of formaldehyde (CH2O) shown; whal
Web hey guys,in this video, we are going to learn about the lewis structure of ch2o. The last step of drawing the lewis structure of the compound is to connect the atoms with bonds. (1) lewis structure of formaldehyde given is correct. Molecular geometry of ch 2 o. Be sure to include all resonance structures that satisfy the octet rule.
[Solved] Draw the Lewis structure of formaldehyde (H2C
Web i quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). First find number of valence electrons: When the question is first opened, a box with a plus symbol is shown below the question. Next draw out the framework of the molecule: Web this problem has been solved!
Lewis Structure Formaldehyde Octet Rule Molecule Chemistry, PNG
It is a colorless gas with a pungent odor. (3) distribute the remaining electrons throughout the molecule, keeping in mind the duet and. Yes, covalent bonds come in pairs which are represented by lines in lewis structures. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4. C neighbors the other atoms,.
Web I Quickly Take You Through How To Draw The Lewis Structure Of Ch2O (Methanal Or Formaldehyde).
Each atom in the molecule has a complete outer shell full of electrons. The last step of drawing the lewis structure of the compound is to connect the atoms with bonds. View the full answer step 2. Search for how many more electrons are required to stabilize the octet.
Lewis Diagram Of Formaldehyde (Ch₂O) The Lewis Diagram Of A Molecule Can Be Constructed Using The Following Stepwise Procedure:
For further queries comment down below. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Eight of these electrons are tied up in bonds.
Lewis Structure Is A Pictorial Representation Of The Atoms In The Molecules, Their Bonds, And Lone Pairs Of Electrons.
Be sure to include all resonance structures that satisfy the octet rule. Formaldehyde (hcho) contains two hydrogen atoms, one carbon atom and one oxygen atom. (1) lewis structure of formaldehyde given is correct. Web formaldehyde lewis structure.
Draw The Lewis Structure For The Formaldehyde (Ch2O) Molecule.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web lewis structure is a simplified diagram which shows the bonding of atoms in a molecule. The molecular geometry of ch 2 o is trigonal planar because the central carbon atom has no lone pair and is attached to the two hydrogen atoms and one oxygen atom through two single bonds. First find number of valence electrons:





![[Solved] Draw the Lewis structure of formaldehyde (H2C](https://media.cheggcdn.com/study/9a8/9a833b2e-204d-4ea7-844b-b668b9777639/image.jpg)