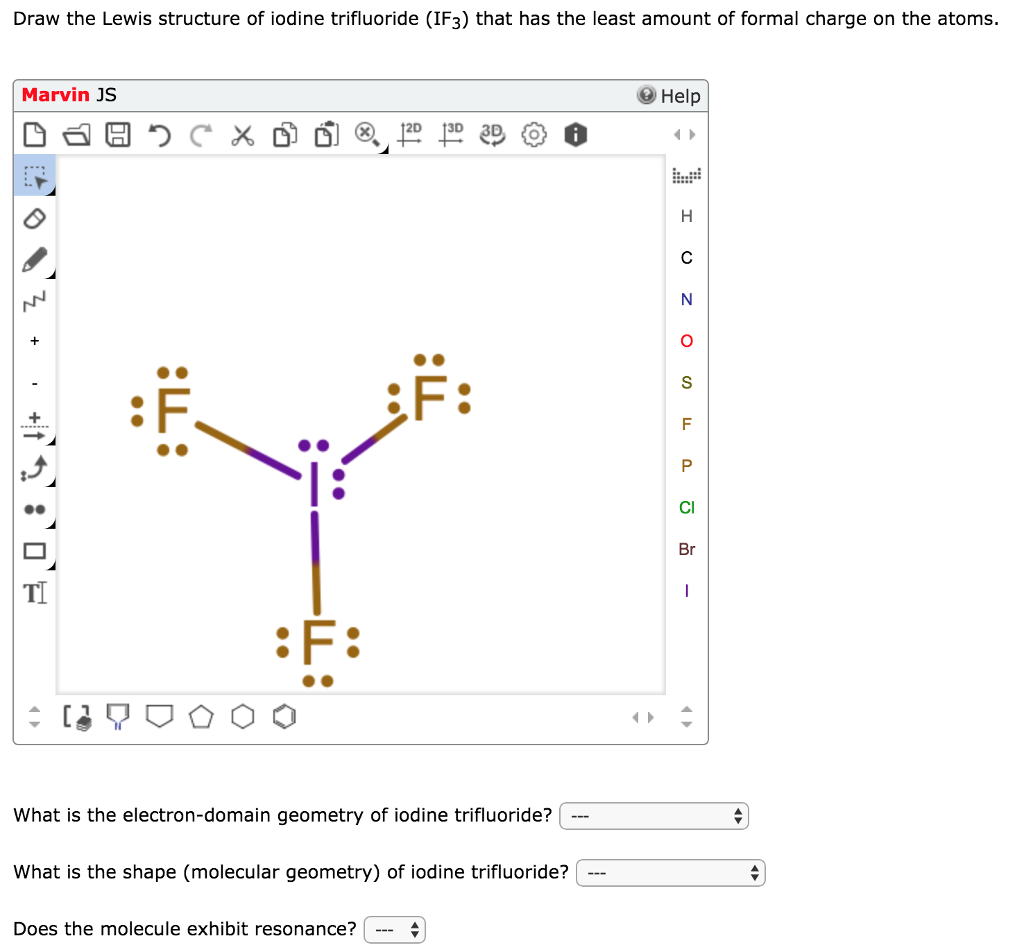
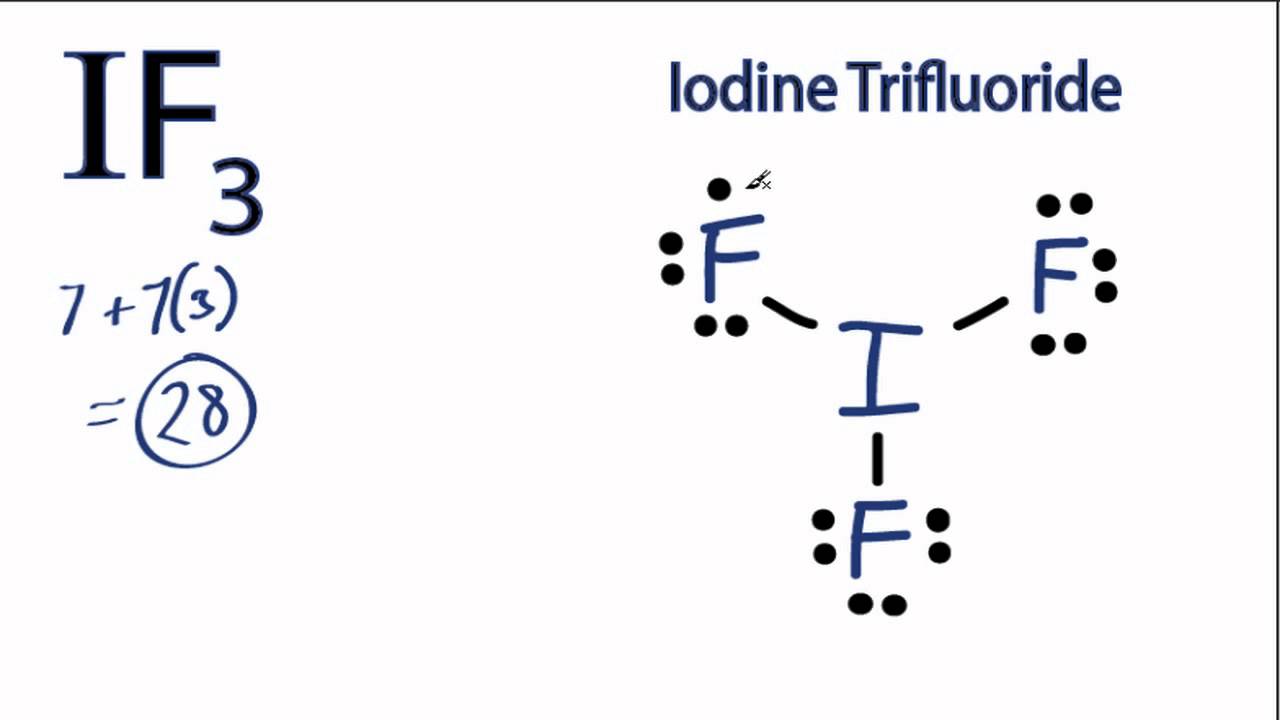
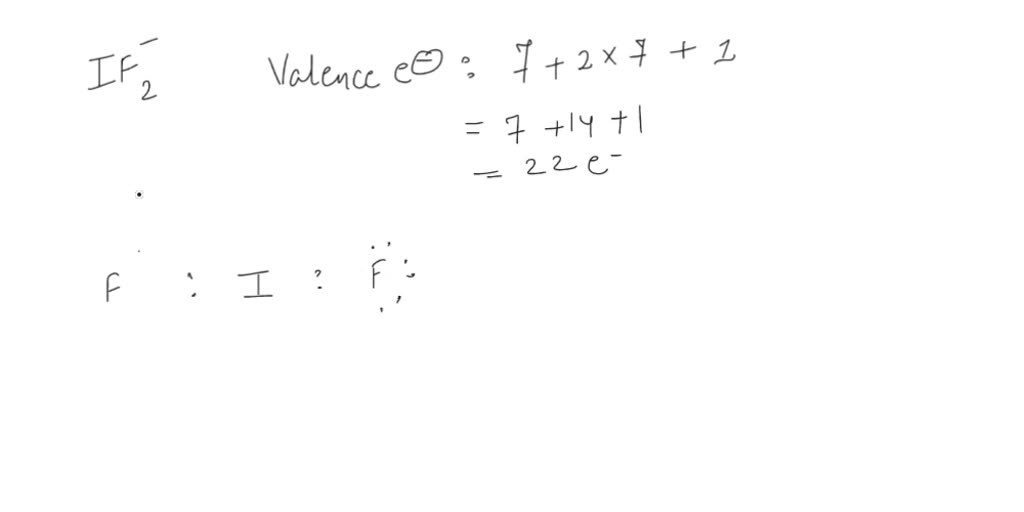Draw The Lewis Structure For The Iodine Difluoride Ion
Draw The Lewis Structure For The Iodine Difluoride Ion - Since it's an ion, we. The structure of hydrogen has to be drawn. Where, v= no of valance electrons, b= no of. Draw lewis structures for covalent compounds. The negative charge on this ion indicates that it accepts an additional electron to form this structure. Follow these simple steps to draw lewis dot structures: Draw the lewis structure for the iodine difluoride (if,) ion. The electronic configuration of kr four d. Count the total number of valence electrons: In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining.
The central atom in lewis structures is usually the least electronegative. Hydrogen has an elected configuration. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Here’s the best way to solve it. Calculate the total number of valence electrons present in the iodine. Iodine (i) has 7 valence electrons, and each fluorine (f) atom has 7 valence electrons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. Iodide is the name of the person. Since it's an ion, we.
Iodide is the name of the person. Web drawing lewis dot structures and resonance structures. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Draw the lewis structure for the iodine difluoride (if,) ion. Where, v= no of valance electrons, b= no of. The central atom in lewis structures is usually the least electronegative. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. Here’s how to approach this question. Web iodine gets the electron. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
IF2 Lewis Structure How to Draw the Lewis Structure for IF2 YouTube
Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. Here, the electrons of iodine would be distributed in the following way to complete the octet of the connected groups: Since it's an ion, we. Follow these simple steps to draw lewis dot structures: Web iodine gets the electron.
Lewis Dot Diagram Iodine
Here, the electrons of iodine would be distributed in the following way to complete the octet of the connected groups: A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Where, v= no of valance electrons, b= no of. Since it's an ion, we. Hydrogen has an elected configuration.
SOLVED Draw the Lewis structure for the iodine difluoride (IF2 ion
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The structure of hydrogen has to be drawn. Iodide is the name of the person. Calculate the total number of valence electrons present in the iodine. Here’s the best way to solve it.
Lewis Structure For Iodine
Since it's an ion, we. Draw the atoms on paper and put dots. Draw the lewis structure for the iodine difluoride (if,) ion. This widget gets the lewis structure of chemical compounds. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or.
SOLVED Draw the Lewis structure for the iodine difluoride ([Fz) ion.
Here’s the best way to solve it. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Since it's an ion, we. Draw lewis structures for covalent compounds. Draw the atoms on paper and put dots.
Solved Draw the Lewis structure for the iodine difluoride
Web may 23, 2023 by jay rana. Here’s how to approach this question. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web iodine gets the electron. This widget gets the lewis structure of chemical compounds.
Iodine Electron Configuration (I) with Orbital Diagram
Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Iodine (i) has 7 valence electrons, and each fluorine (f) atom has 7 valence electrons. Count the total number of valence electrons: Follow these simple steps to draw.
Lewis Structure For Iodine
Where, v= no of valance electrons, b= no of. Here’s the best way to solve it. Calculate the total number of valence electrons present in the iodine. The negative charge on this ion indicates that it accepts an additional electron to form this structure. Web drawing lewis dot structures and resonance structures.
Lewis dot structure for IO2F2− YouTube
Hydrogen has an elected configuration. Since it's an ion, we. Where, v= no of valance electrons, b= no of. Draw the lewis structure for the iodine difluoride (if,) ion. The central atom in lewis structures is usually the least electronegative.
(Get Answer) Draw The Lewis Structure For The Iodine Difluoride (IF 7
Iodide is the name of the person. Since it's an ion, we. Web may 23, 2023 by jay rana. Here, the electrons of iodine would be distributed in the following way to complete the octet of the connected groups: Web iodine gets the electron.
Draw The Lewis Structure For The Iodine Difluoride (If,) Ion.
Iodide is the name of the person. Here’s the best way to solve it. Web draw lewis structures for ionic compounds. Here, the electrons of iodine would be distributed in the following way to complete the octet of the connected groups:
Since It's An Ion, We.
The electronic configuration of kr four d. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses. Calculate the total number of valence electrons.
19K Views 3 Years Ago.
The structure of hydrogen has to be drawn. Draw the atoms on paper and put dots. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web drawing lewis dot structures and resonance structures.
Iodine (I) Has 7 Valence Electrons, And Each Fluorine (F) Atom Has 7 Valence Electrons.
Hydrogen has an elected configuration. Count the total number of valence electrons: Web iodine gets the electron. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or.