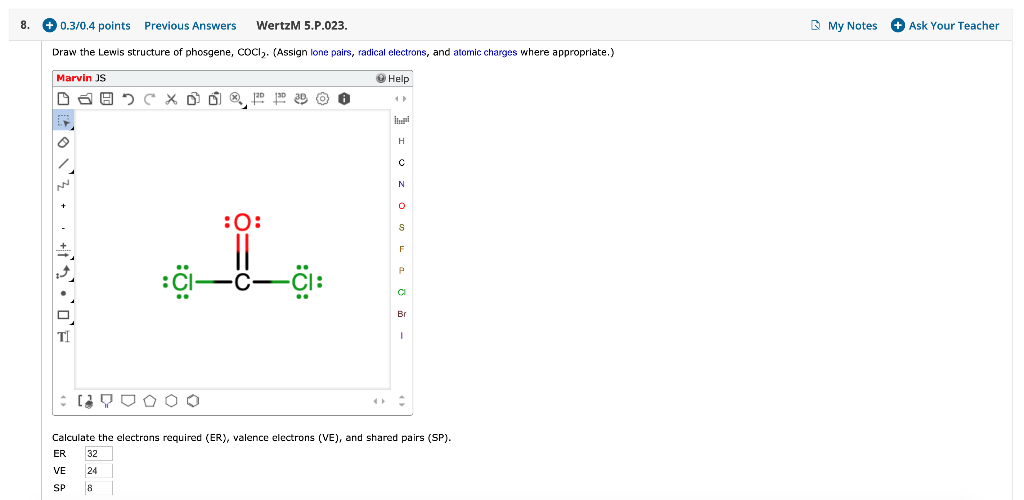
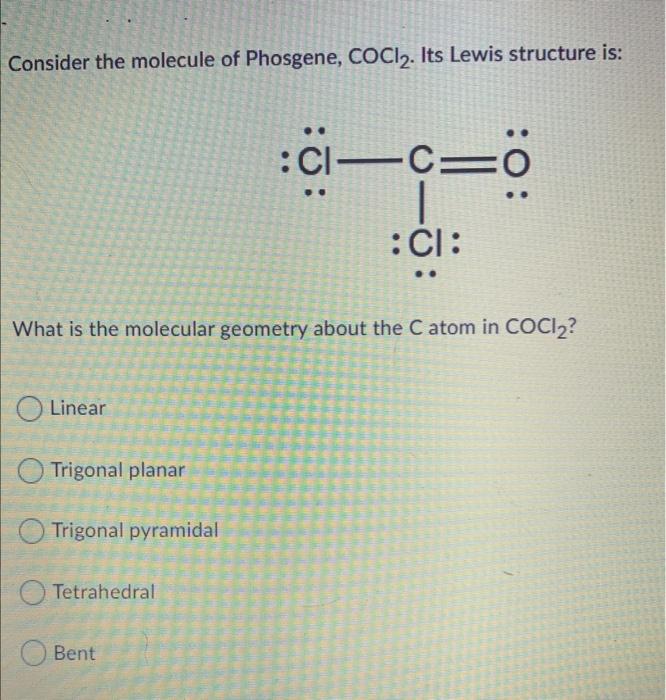
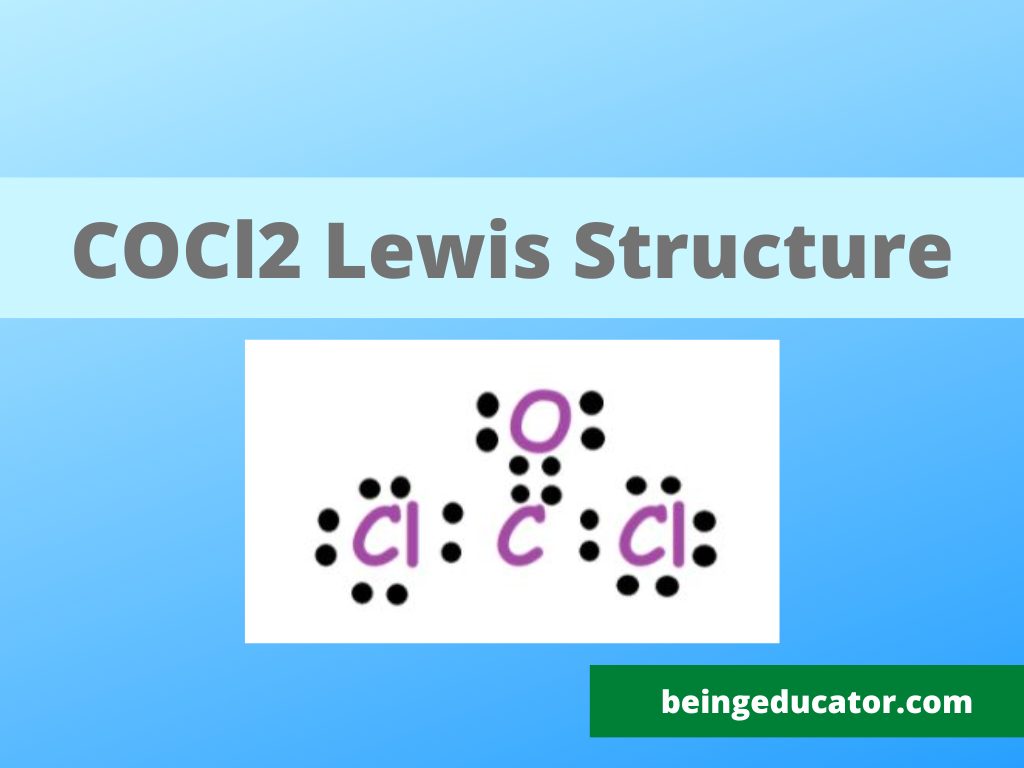
Draw The Lewis Structure For The Phosgene Cocl2 Molecule
Draw The Lewis Structure For The Phosgene Cocl2 Molecule - Web to properly draw the cocl 2 lewis structure, follow these steps: Web a student proposes the following lewis structure for the phosgene cocl2 molecule. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. (assign lone pairs, radical electrons, and atomic charges where appropriate.). Assign a formal charge to each atom in the student's lewis structure. Phosgene is a planar molecule as predicted by vsepr theory. Web the following procedure can be used to draw lewis structure for simple molecules. Draw the lewis structure total valence electrons lone pairs of electrons (central atom) bond pairs. The lewis structure represents the most stable and probable structure. The c=o distance is 1.18 å, the c−cl distance is 1.74 å and the cl−c−cl angle.
Draw the lewis structure total valence electrons lone pairs of electrons (central atom) bond pairs. #2 next, indicate lone pairs on the atoms. Web in the lewis structure for cocl 2 there are a total of 24 valence electrons. Web a student proposes the following lewis structure for the phosgene cocl2 molecule. The c=o distance is 1.18 å, the c−cl distance is 1.74 å and the cl−c−cl angle. Web to properly draw the cocl 2 lewis structure, follow these steps: Web structure and basic properties. Draw the lewis structure of phosgene, cocl2. Phosgene is a planar molecule as predicted by vsepr theory. Web structure, properties, spectra, suppliers and links for:
Web structure, properties, spectra, suppliers and links for: Phosgene is a planar molecule as predicted by vsepr theory. (assign lone pairs, radical electrons, and atomic charges where appropriate.). Copy sheet of paper on top of another sheet. Drawing and evaluating phosgene (coci2 a. Web the lewis structure of cocl2, also known as carbonyl chloride or phosgene, is a representation of its molecular structure using lewis symbols and lines to. Web to properly draw the cocl 2 lewis structure, follow these steps: 23k views 2 years ago. Web a student proposes the following lewis structure for the phosgene cocl2 molecule. Draw the lewis structure of phosgene, cocl2.
The Lewis Structure of COCl2 [with free study guide and video]
Phosgene is a planar molecule as predicted by vsepr theory. #2 next, indicate lone pairs on the atoms. Web the following procedure can be used to draw lewis structure for simple molecules. 23k views 2 years ago. Copy sheet of paper on top of another sheet.
COCl2 Molecular Geometry, Bond Angles (Phosgene) YouTube
Web a student proposes the following lewis structure for the phosgene cocl2 molecule. Web the lewis structure of cocl2, also known as carbonyl chloride or phosgene, is a representation of its molecular structure using lewis symbols and lines to. Phosgene is a planar molecule as predicted by vsepr theory. Copy sheet of paper on top of another sheet. 23k views.
Cocl2 Lewis Dot Structure Draw Easy
Web to properly draw the cocl 2 lewis structure, follow these steps: Drawing and evaluating phosgene (coci2 a. Phosgene is a planar molecule as predicted by vsepr theory. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. #1 draw a rough sketch of the structure.
Solved Draw the Lewis structure of phosgene, COCl2. (Assign
The c=o distance is 1.18 å, the c−cl distance is 1.74 å and the cl−c−cl angle. Copy sheet of paper on top of another sheet. Web in the lewis structure for cocl 2 there are a total of 24 valence electrons. Drawing and evaluating phosgene (coci2 a. Web structure and basic properties.
COCl2 Lewis Structure How to Draw the Lewis Structure for COCl2 YouTube
Web to properly draw the cocl 2 lewis structure, follow these steps: The c=o distance is 1.18 å, the c−cl distance is 1.74 å and the cl−c−cl angle. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. Assign a formal charge to each atom in the student's lewis structure. #2 next,.
Solved Consider the molecule of Phosgene, COCl2. Its Lewis
Web the following procedure can be used to draw lewis structure for simple molecules. Web to properly draw the cocl 2 lewis structure, follow these steps: You'll need to form a double bond between the carbon and oxygen to complete the octet on the. Web structure and basic properties. 23k views 2 years ago.
Lewis StructuresDot Structures Drawn for phosgene (COCl2) Ex. 6
Phosgene is a planar molecule as predicted by vsepr theory. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. Web to properly draw the cocl 2 lewis structure, follow these steps: 23k views 2 years ago. (assign lone pairs, radical electrons, and atomic charges where appropriate.).
Phosgene having a chemical formula of COCl2, is a polyatomic molecule
Drawing and evaluating phosgene (coci2 a. Phosgene is a planar molecule as predicted by vsepr theory. 23k views 2 years ago. See the diagram for the lewis structure of cocl2 (phosgene gas). Draw the lewis structure of phosgene, cocl2.
COCl2 Lewis Structure Hybridization Polarity Molecular Geometry
See the diagram for the lewis structure of cocl2 (phosgene gas). Phosgene is a planar molecule as predicted by vsepr theory. Web structure, properties, spectra, suppliers and links for: #1 draw a rough sketch of the structure. Draw the lewis structure of phosgene, cocl2.
Cocl2 Lewis Structure Shape Draw Easy
The lewis structure represents the most stable and probable structure. Draw the lewis structure of phosgene, cocl2. Web to properly draw the cocl 2 lewis structure, follow these steps: See the diagram for the lewis structure of cocl2 (phosgene gas). You'll need to form a double bond between the carbon and oxygen to complete the octet on the.
Web In The Lewis Structure For Cocl 2 There Are A Total Of 24 Valence Electrons.
Drawing and evaluating phosgene (coci2 a. Phosgene is a planar molecule as predicted by vsepr theory. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. Web to properly draw the cocl 2 lewis structure, follow these steps:
23K Views 2 Years Ago.
Copy sheet of paper on top of another sheet. Assign a formal charge to each atom in the student's lewis structure. Web structure, properties, spectra, suppliers and links for: Web structure and basic properties.
#2 Next, Indicate Lone Pairs On The Atoms.
#1 draw a rough sketch of the structure. The lewis structure represents the most stable and probable structure. Draw the lewis structure total valence electrons lone pairs of electrons (central atom) bond pairs. See the diagram for the lewis structure of cocl2 (phosgene gas).
An Explanation Of The Molecular Geometry For The Cocl2 (Phosgene) Including A.
The c=o distance is 1.18 å, the c−cl distance is 1.74 å and the cl−c−cl angle. Draw the lewis structure of phosgene, cocl2, which was used as a chemical weapon during world war i. Draw the lewis structure of phosgene, cocl2. (assign lone pairs, radical electrons, and atomic charges where appropriate.).
![The Lewis Structure of COCl2 [with free study guide and video]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/COCl2-lewis-structure.jpg)