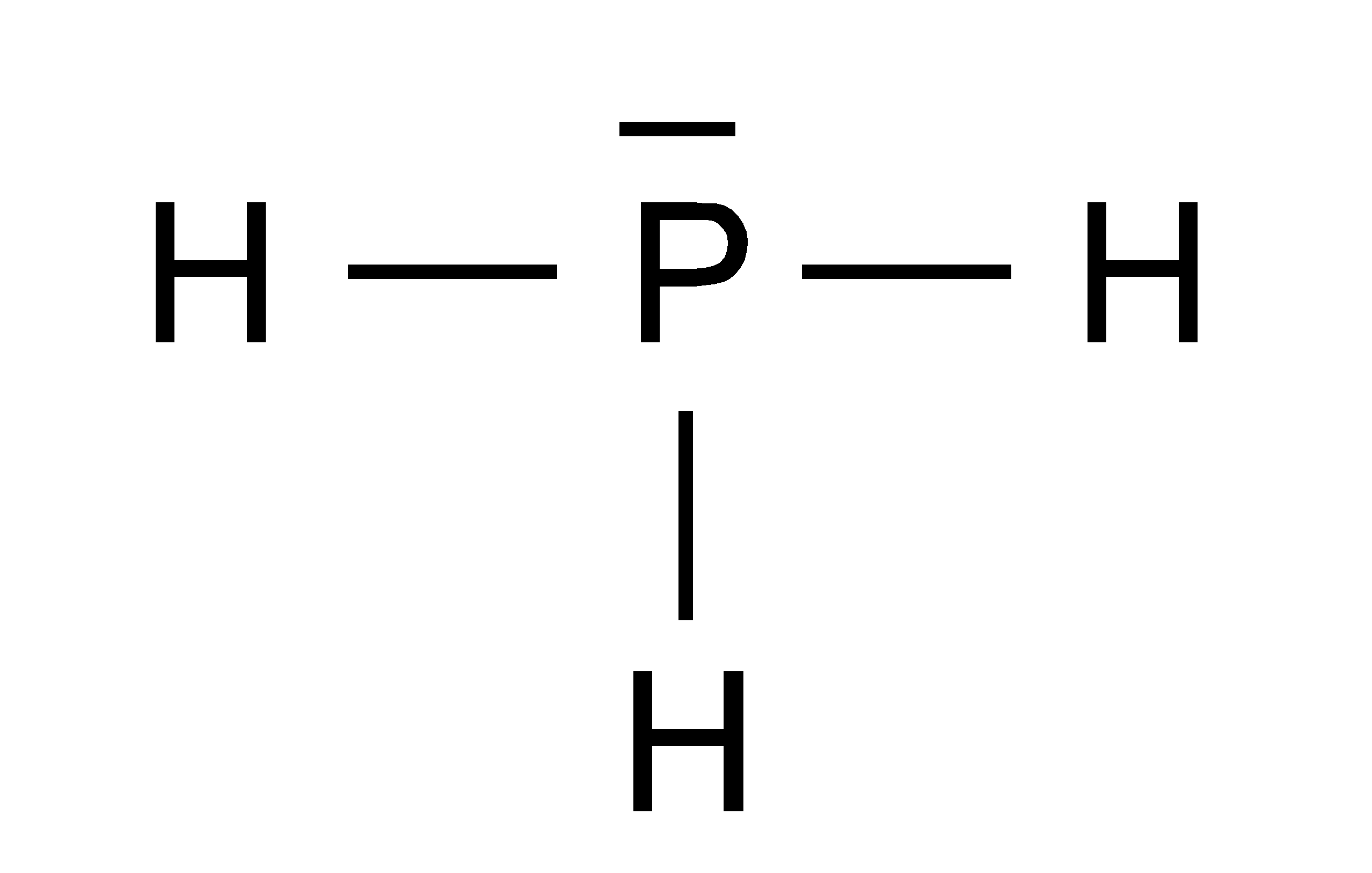
Draw The Lewis Structure Of Ph3
Draw The Lewis Structure Of Ph3 - Therefore, it has only one electron on top. (valence electrons are the number of electrons present in the. Providing for three open bonding sites. Web draw the lewis structure for ph3 (p is the central atom) and answer the questions below. In the ph 3 lewis structure (and all lewis structures) hydrogen goes on the outside. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. The molecular geometry and shape of the ph3 molecule is a trigonal pyramid. Part a draw the lewis structure of ph3 to add lone pairs, click the button before clicking on the molecule. Web steps of drawing lewis structure of ph 3. Web from the lewis molecular structure of ph3, we have seen the phosphorous atom has five valence electrons.
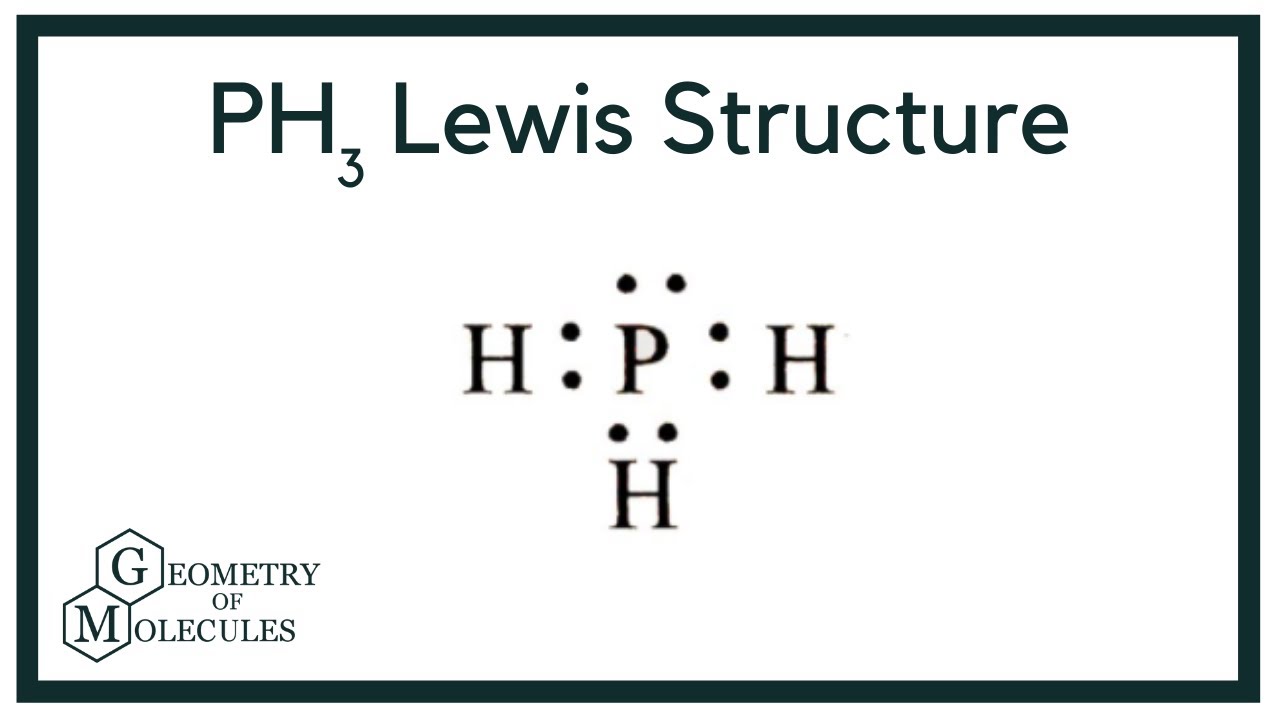
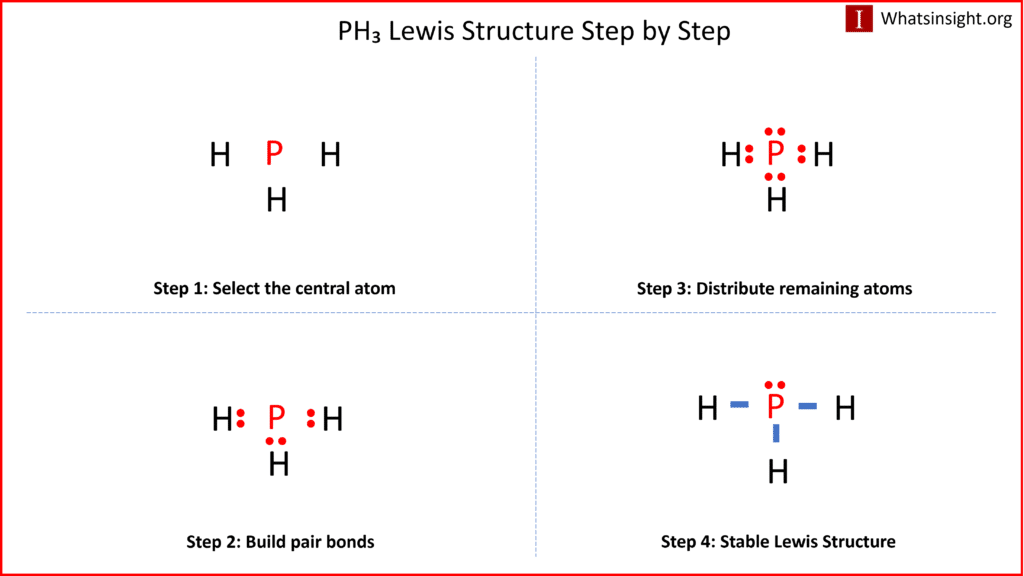
#2 next, indicate lone pairs on the atoms. In the structure of phosphane, we can see that there are 3 atoms of hydrogen element one phosphorus element atom is present. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. Web * phosphorus requires a full octet of electrons, and brings 5 with it.* each hydrogen brings 1 electron* this allows for three single bonds and one lone pair. 4 + (3 × 6) + 2 = 24 electrons. #1 draw a rough sketch of the structure. Therefore, it has a dot digram in the red of two electrons on top and one electron on each side. Web how to draw lewis structure for ph3? Phosphosus has an electron configuration of [ne] 2s^2 2p^3. For ph3, this sums up to 8 valence electrons.
Calculate the total number of valence electrons. To add bonds connect atoms with a line draw the molecule by placing atoms on the grid and connecting them with bonds. Web * phosphorus requires a full octet of electrons, and brings 5 with it.* each hydrogen brings 1 electron* this allows for three single bonds and one lone pair. The ph3 lewis structure has 8. Therefore, it has only one electron on top. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. There are several steps to draw the lewis structure of ph 3. Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure: This is the lewis structure we drew earlier. Web how to draw lewis structure for ph3?
PH3 Lewis Structure How to Draw the Lewis Structure for PH3 YouTube
Those steps are explained in detail in this tutorial. There are several steps to draw the lewis structure of ph 3. This problem has been solved! Providing for three open bonding sites. Phosphorus (p) contributes 5 valence electrons, and each hydrogen (h) contributes 1.
PH3 Lewis Structure (Phosphine) YouTube
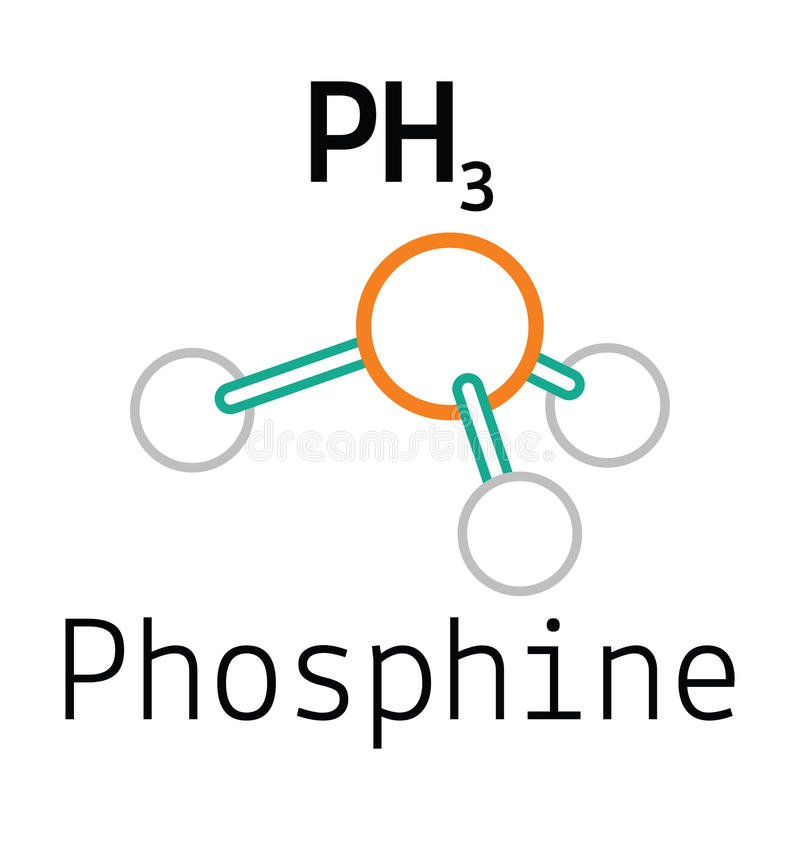
There are several steps to draw the lewis structure of ph 3. Web draw a trial structure by putting electron pairs around every atom until each gets an octet. The molecular geometry and shape of the ph3 molecule is a trigonal pyramid. Coming to the contribution of. Web draw the lewis structure for ph3 (p is the central atom) and.
Draw The Lewis Structure Of Ph3
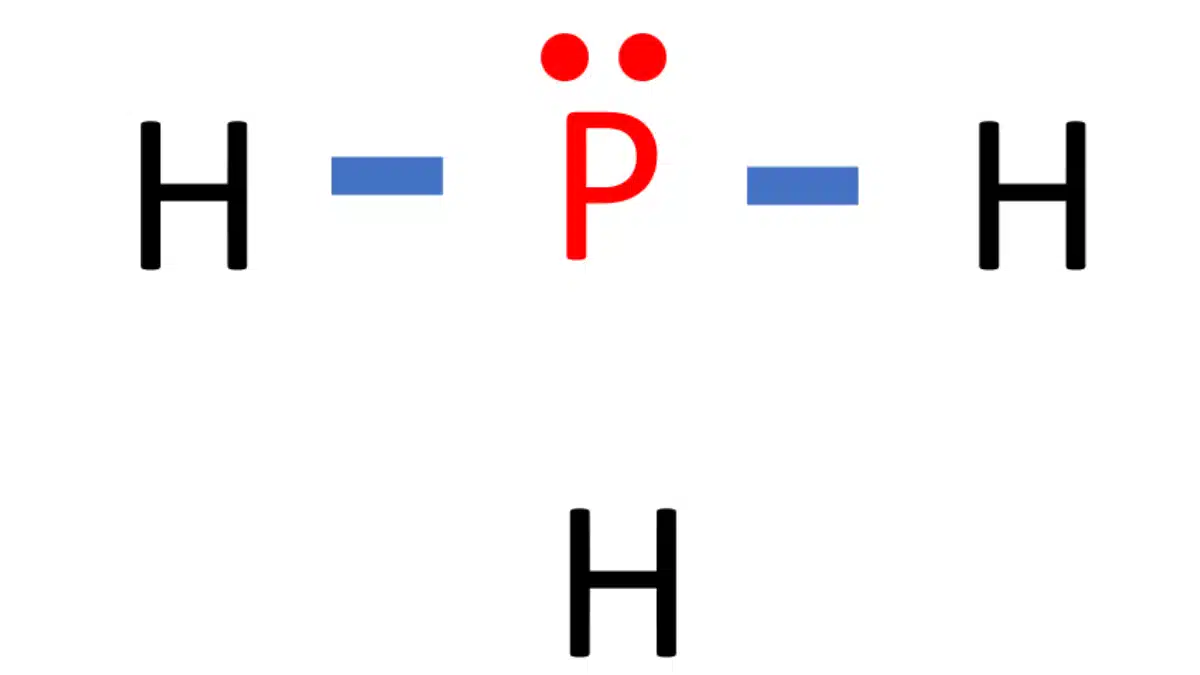
Web the lewis structure for ph 3 is similar to nh 3. Phosphorus atom is in the centre forming single bonds with three hydrogen atoms and also has a lone pair of electrons in its lewis structure. This problem has been solved! Web from the lewis molecular structure of ph3, we have seen the phosphorous atom has five valence electrons..
Draw The Lewis Structure Of Ph3 Drawing.rjuuc.edu.np
During the bonding process, phosphorous is surrounded by three hydrogen atoms, and each is connected by a single bond. Calculate the total number of valence electrons. Hydrogen has an electron configuration of 1s^1. 1 p + 3 h =. Web from the lewis molecular structure of ph3, we have seen the phosphorous atom has five valence electrons.
Draw the Lewis dot structure for PH3 YouTube
Web how to draw lewis structure for ph3? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For the ph3 lewis structure we first count the valence electrons for the ph3 molecule using the periodic table. Draw the lewis structure of ph3. To add bonds connect atoms with a line draw the molecule.
PH3 Lewis Structure in four simple steps What's Insight
This is the lewis structure we drew earlier. The ph3 lewis structure has 8. 1 p + 3 h =. Phosphosus has an electron configuration of [ne] 2s^2 2p^3. 1×5 + 3×1 = 8.
PH3 Lewis Structure in four simple steps What's Insight
Web 6 steps to draw the lewis structure of ph3 step #1: Coming to the contribution of. For ph3, this sums up to 8 valence electrons. Hydrogen has an electron configuration of 1s^1. Phosphorus (p) contributes 5 valence electrons, and each hydrogen (h) contributes 1.
PH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle and
(a) how many hydrogen atoms are in the molecule? The carbon—carbon bonds are labeled 1, 2, and 3. Providing for three open bonding sites. Phosphorus atom is in the centre forming single bonds with three hydrogen atoms and also has a lone pair of electrons in its lewis structure. For the ph3 lewis structure we first count the valence electrons.
Ph3 Lewis Structure Shape
1 p + 3 h =. Web from the lewis molecular structure of ph3, we have seen the phosphorous atom has five valence electrons. Phosphorus (p) contributes 5 valence electrons, and each hydrogen (h) contributes 1. Each h atom has a full valence shell of 2 electrons. During the bonding process, phosphorous is surrounded by three hydrogen atoms, and each.
Ph3 Lewis Structure Shape
Web * phosphorus requires a full octet of electrons, and brings 5 with it.* each hydrogen brings 1 electron* this allows for three single bonds and one lone pair. Here, the given molecule is ph3. Here’s the best way to solve it. Identify the number of valence electrons for each atom in the molecule. Draw the lewis dot structure for.
Adding The Remaining 4 Electrons To The Oxygen (As Two Lone Pairs) Gives The Following Structure:
This problem has been solved! Part a draw the lewis structure of ph3 to add lone pairs, click the button before clicking on the molecule. Here’s the best way to solve it. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell.
In The Ph 3 Lewis Structure (And All Lewis Structures) Hydrogen Goes On The Outside.
For ph3, this sums up to 8 valence electrons. Three pairs will be used in the chemical bonds. Web draw a trial structure by putting electron pairs around every atom until each gets an octet. Draw the lewis structure of ph3.
Phosphorus (P) Contributes 5 Valence Electrons, And Each Hydrogen (H) Contributes 1.
Welcome back to geometry of molecules where we make chemistry fun and easy. Calculate the total number of valence electrons. The final answer must have this number of electrons‼! In the structure of phosphane, we can see that there are 3 atoms of hydrogen element one phosphorus element atom is present.
Therefore, It Has A Dot Digram In The Red Of Two Electrons On Top And One Electron On Each Side.
See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. To add bonds connect atoms with a line draw the molecule by placing atoms on the grid and connecting them with bonds. #1 draw a rough sketch of the structure. A) draw a lewis structure for ph3 in which all atoms except hydrogen obey the octet rule.