Draw The Lewis Structures For Resonance Forms Of Acetamide
Draw The Lewis Structures For Resonance Forms Of Acetamide - What resonance structure can account for the planar geometry about the nitrogen atom? Breaking the octet rule ; We will bond central carbon atom to the another carbon atom, nitrogen atom and to the oxygen atom. We will put the double bond between c and o atom. Experiments show that the geometry about each interior atom in acetamide is nearly planar. Therefore, its geometry is trigonal planar. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Resonance occurs due to the delocalization of electrons between the carbon and nitrogen atoms. Molecules with only single bonds never show resonance. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar.
Therefore, its geometry is trigonal planar. We will put the double bond between c and o atom. Web we have two resonance structure for this molecule. Added jun 9, 2014 by webtester in chemistry. Find more chemistry widgets in wolfram|alpha. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Molecules with only single bonds never show resonance. Web draw the lewis structure for resonance forms of acetamide (ch3conh2) this problem has been solved! Web drawing lewis structures for molecules with one central atom: We will bond central carbon atom to the another carbon atom, nitrogen atom and to the oxygen atom.
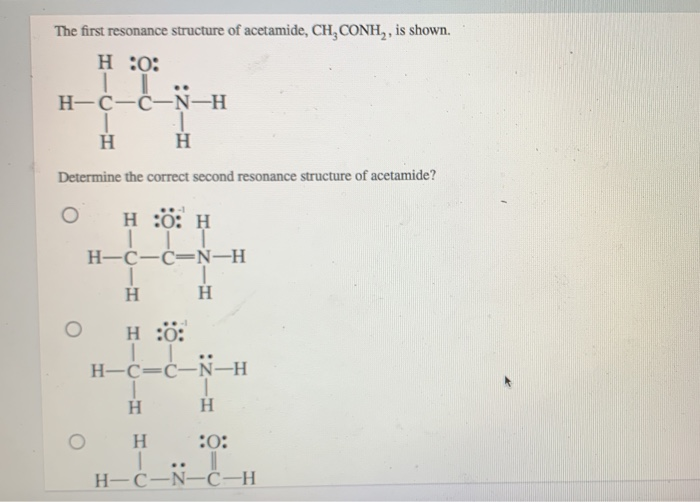
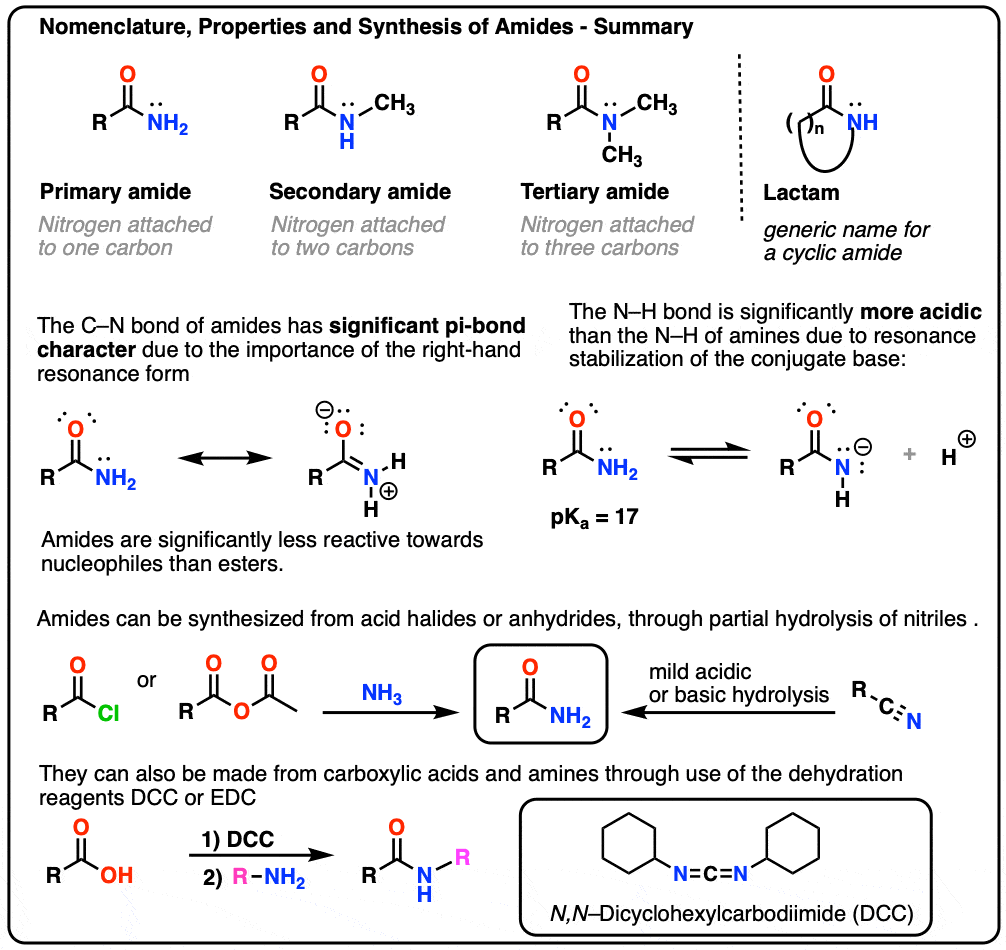
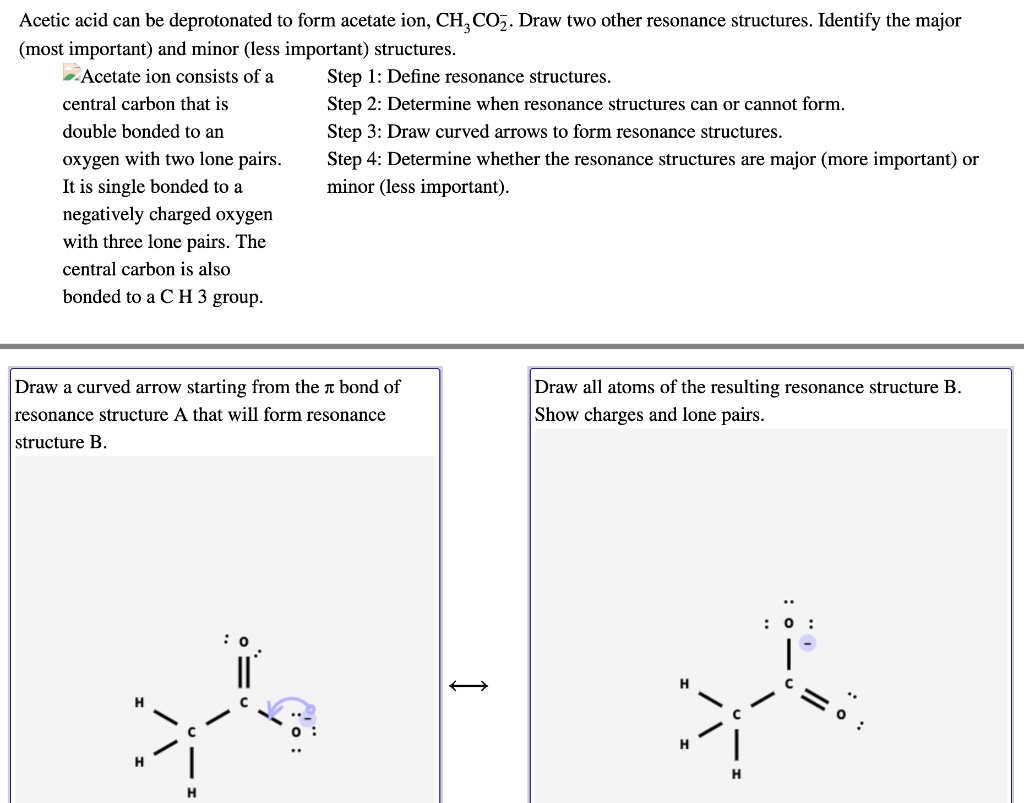
Instead, the actual structure is somewhere in between the structures shown. We will attach two h atoms to the n atom and three h atoms to the terminal c atom. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar. Web draw the lewis structure for acetamide ch 3 conh 2 an organic compound, and determine the geometry about each interior atom. Equivalent lewis structures are called resonance forms. They are used when there is more than one way to place double bonds and lone pairs on atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structures for resonance forms of acetamide. Now, let's determine the geometry about each interior atom: Show the formal charges of all nonhydrogen atoms in the correct structures.
Ch3conh2 Resonance Structures
Web draw the lewis structure for resonance forms of acetamide (ch3conh2) this problem has been solved! Web choose the most favorable lewis structure. Why is this resonance system better? What resonance structure can account for the planar geometry about the nitrogen atom? We will bond central carbon atom to the another carbon atom, nitrogen atom and to the oxygen atom.
draw the lewis structures for resonance forms of acetamide
Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar. Include all hydrogen atoms and. Molecules with only single bonds never show resonance. Draw the lewis structures for resonance forms of acetamide. Which resonance structure can account for the planar geometry about the nitrogen atom?
Solved Draw The Lewis Structure For Acetamide Ch My XXX Hot Girl
What resonance structure can account for the planar geometry about the nitrogen atom? Web draw the lewis structure for resonance forms of acetamide (ch3conh2) this problem has been solved! They are used when there is more than one way to place double bonds and lone pairs on atoms. Draw the lewis structure for acetamide (ch3conh2) and determine the geometry about.
Draw The Lewis Structures For Resonance Forms Of Acetamide Printable
Draw the lewis structures for resonance forms of acetamide. Resonance occurs due to the delocalization of electrons between the carbon and nitrogen atoms. Draw the lewis structure for acetamide (ch3conh2), an organic compound, and determine the geometry about each interior atom. Resonance only occurs when a molecule has at least one double bond. You'll get a detailed solution from a.
Draw The Lewis Structures For Resonance Forms Of Acetamide Printable
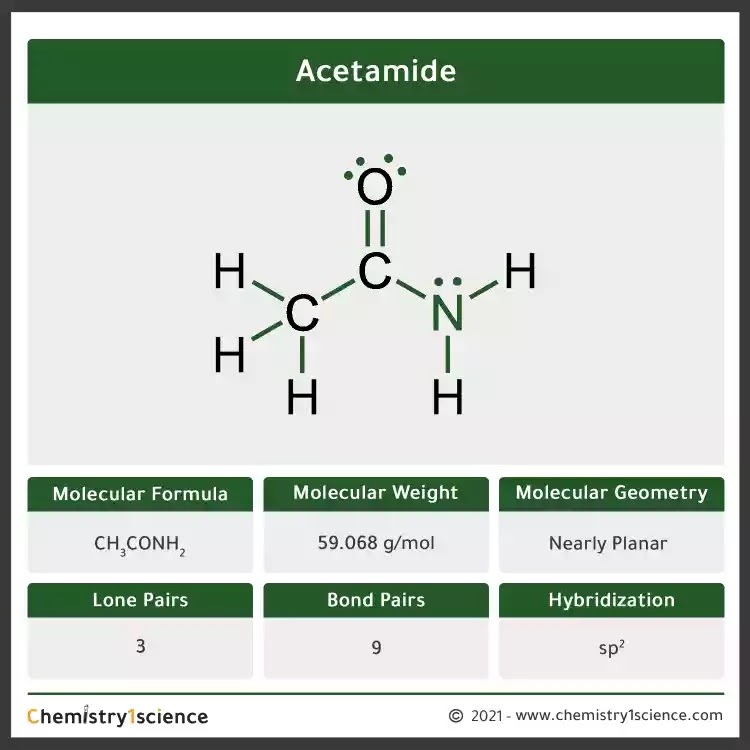
First, we need to draw the lewis structure for acetamide (ch3conh2). Using formal charges to determine how many bonds to make, a different perspective. Drawing lewis structures for bf3, pf3 and brf3; Acetamide has the formula ch3conh2. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar.
draw the lewis structures for resonance forms of acetamide
Step 2/4 next, we need to identify the areas where we can move electron density to create a new. Experiments show that the geometry about each interior atom in acetamide is nearly planar. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structures for resonance forms of acetamide. What resonance.
Draw The Lewis Structures For Resonance Forms Of Acetamide Printable
Breaking the octet rule ; Now, let's determine the geometry about each interior atom: Added jun 9, 2014 by webtester in chemistry. Draw the molecule by placing atoms on the grid and connecting them with bonds. Equivalent lewis structures are called resonance forms.
[Solved] Following is the structural formula of acetamide. (a) Complete
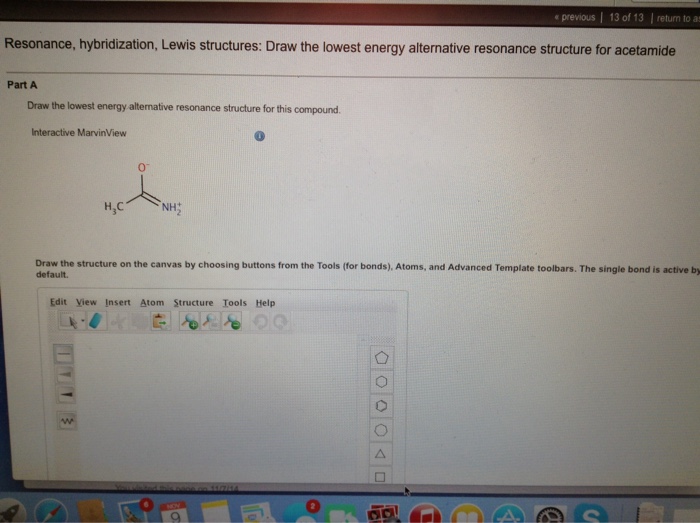
Draw the molecules by placing atoms on the grid and connecting them with bonds. Draw the lewis structures for resonance forms of acetamide. Draw the lewis structure for acetamide (ch3conh2), an organic compound, and determine the geometry about each interior atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the.
SOLVED 'Write another resonance structure for acetamide Acetamide'
This widget gets the lewis structure of chemical compounds. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar. Include all hydrogen atoms and nonbonding electrons. 26 views 1 month ago #chemistry q&a. Draw the lewis structure for acetamide (ch3conh2) and determine the geometry about each interior atom.
SOLVED Draw the Lewis structure for acetamide (CH3CONH2), an organic
Remember that the molecule does not actually switch between these structures. C 2 h 5 no. Find more chemistry widgets in wolfram|alpha. Draw the molecule by placing atoms on the grid and connecting them with bonds. We will put the double bond between c and o atom.
Draw The Lewis Structure For Acetamide (Ch3Conh2), An Organic Compound, And Determine The Geometry About Each Interior Atom.
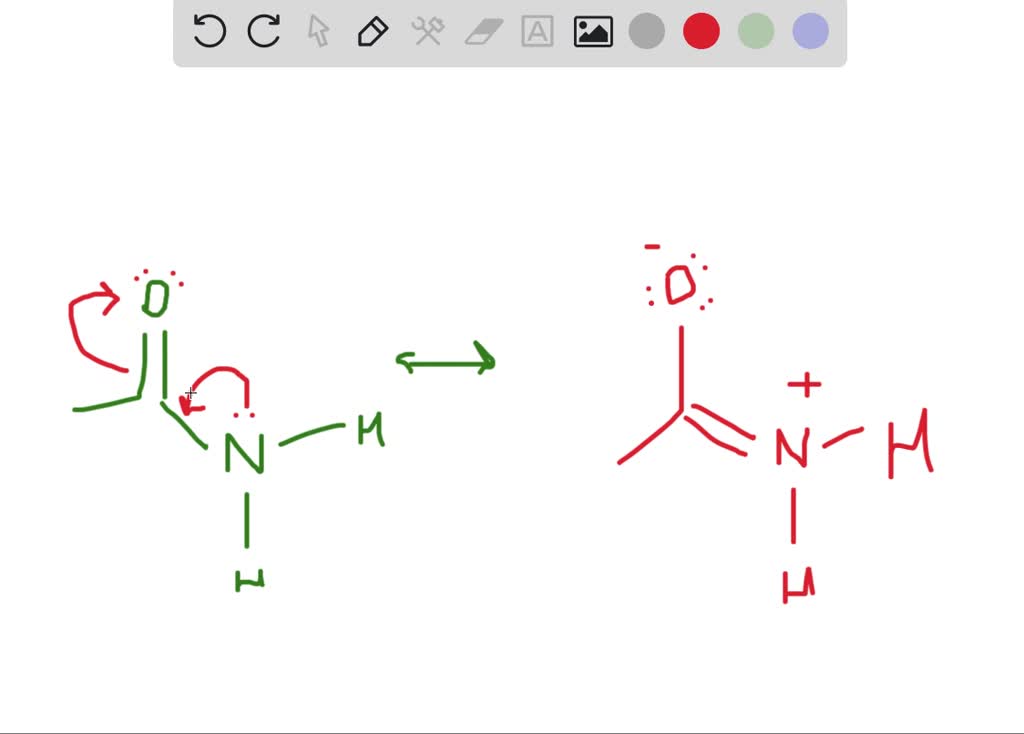
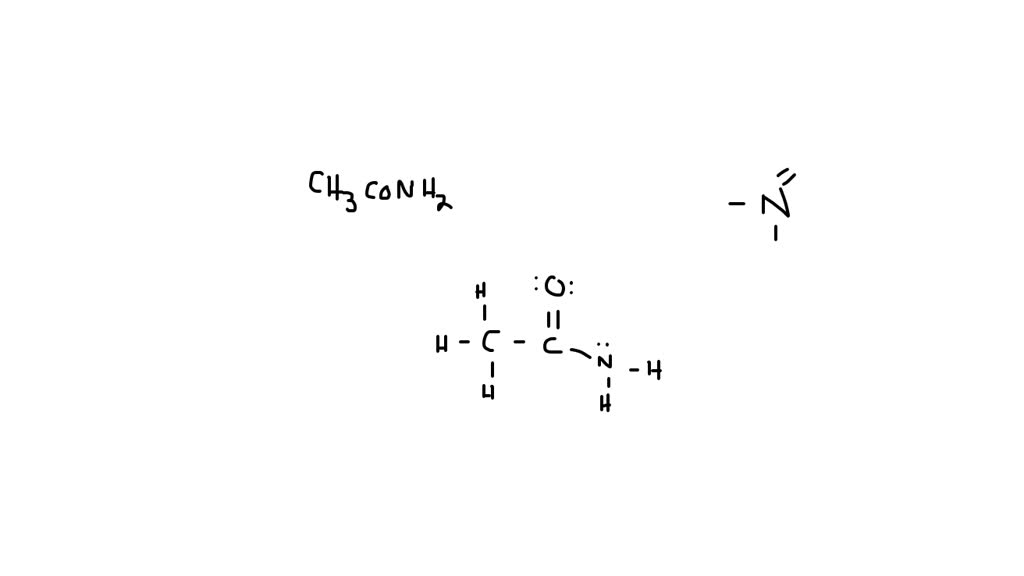
The lewis structure of acetamide (ch3conh2) exhibits resonance, with two resonance forms. Drawing lewis structures for bf3, pf3 and brf3; Web draw the lewis structure for acetamide ch 3 conh 2 an organic compound, and determine the geometry about each interior atom. Step 2/4 next, we need to identify the areas where we can move electron density to create a new.
What Resonance Structure Can Account For The Planar Geometry About The Nitrogen Atom?
What resonance structure can account for the planar geometry about the nitrogen atom? The lewis structure shows that the nitrogen atom has a lone pair of electrons and the oxygen atom is double bonded to the carbon atom. Which resonance structure can account for the planar geometry about the nitrogen atom? What resonance structure can account for the planar geometry about the nitrogen atom?
Web First, We Need To Draw The Lewis Structure Of Acetamide.
Web choose the most favorable lewis structure. Experiments show that the geometry about the nitrogen atom in acetamide is nearly planar. It can be thought of as some average of these structures. We will put the double bond between c and o atom.
Resonance To The Rescue (Again) −/1 Points Once Again, A Single Lewis Structure For A Molecule Failed To Predict Its Actual Structure.
Now, let's determine the geometry about each interior atom: Web a resonance form is another way of drawing a lewis dot structure for a given compound. Draw the molecule by placing atoms on the grid and connecting them with bonds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.