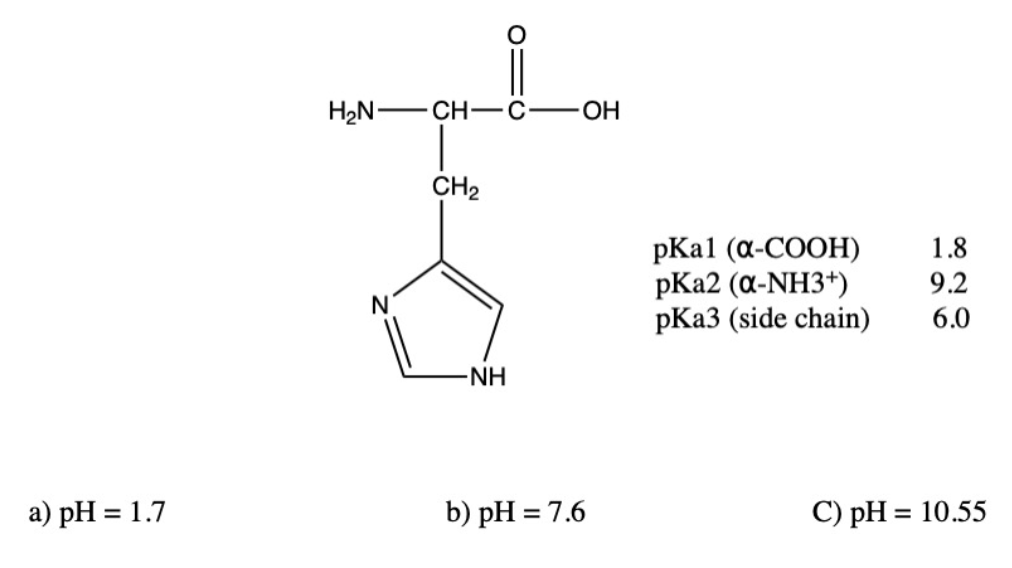
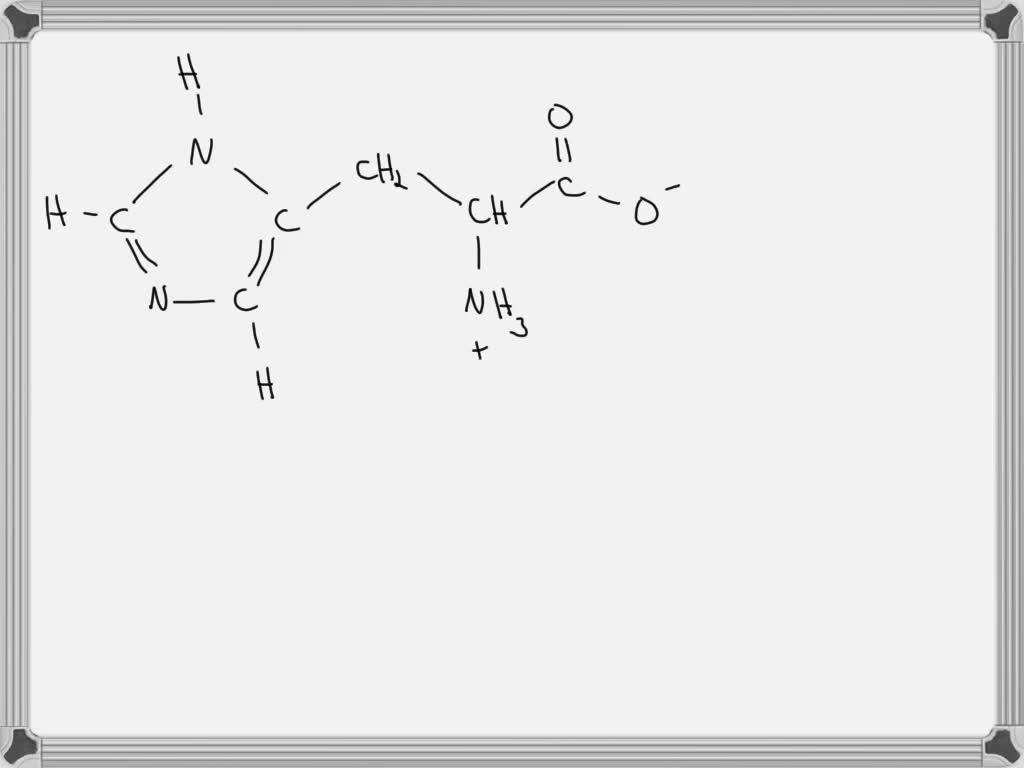
Draw The Predominant Form Of Histidine At Ph 0
Draw The Predominant Form Of Histidine At Ph 0 - Web ph = 0 ph = 7 ph = 11 ho2. At ph = 1.7 there is a equal amount of h3a2 + and h2a+. Describe, briefly, how a mixture of amino. Pka 3.86 nh2 o ho o oh glutamic acid pka 4.25 histidine nh2 o ho n+ n h h pka 6.04 nh2 o ho sh cysteine pka 8.35 lysine nh2 o ho nh3+ pka 10.79 nh2 o ho oh tyrosine pka 10.07 arginine nh2 o ho h n nh2 nh2+ pka 12.48. Draw the predominant form of each amino acid at ph 3.0. Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. This problem has been solved! Web histidine is an essential amino acid that plays a vital role in the biosynthesis of proteins. Web (a) the isoelectric point (pi) of phenylalanine is ph 5.5.
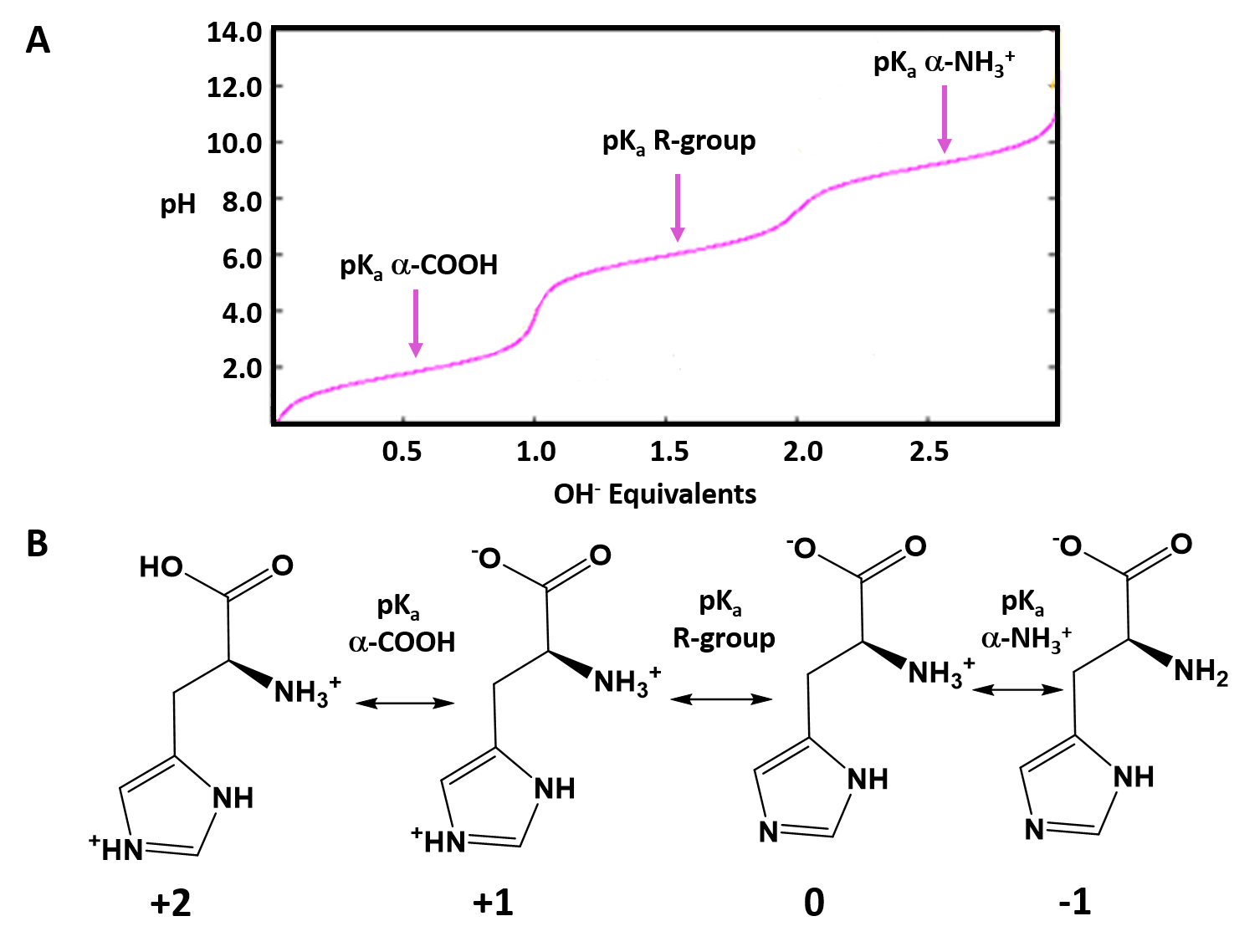
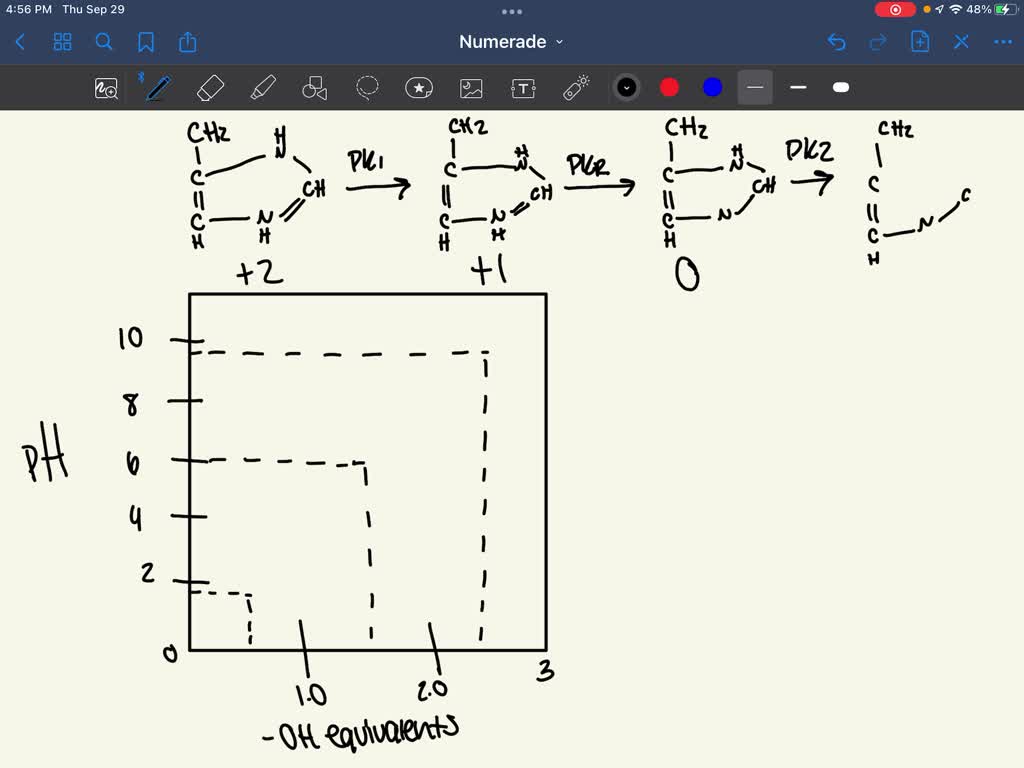
At ph = 6.02 there is equal amounts of h2a+ and ha. Draw each structure and analyze. Draw the predominate form of histidine at physiological ph. Draw histidine in its predominant form at ph = 7.0. Pka 3.86 nh2 o ho o oh glutamic acid pka 4.25 histidine nh2 o ho n+ n h h pka 6.04 nh2 o ho sh cysteine pka 8.35 lysine nh2 o ho nh3+ pka 10.79 nh2 o ho oh tyrosine pka 10.07 arginine nh2 o ho h n nh2 nh2+ pka 12.48. This is because the ph scale measures the concentration of hydrogen ions in a solution, and at low ph, there is an abundance of h⁺. Identify the amino acid with the lowest pl (try to solve this problem by inspecting their structures, rather than performing calculations). Zwitterions problem 8 draw the. Draw the predominant form of histidine at the following ph values. The ph most proteins would encounter).
Here’s the best way to solve it. As the pka is very close to the ph, only small changes in the local environment can change the protonation state of the amino acid. Draw the structures of the major forms of histidine at ph values of 1, 4,7.6, and 11. Web there are only one or two dominant species present at a particular ph. At ph 2 at ph 7.8 at ph 10 would you expect the amino acid tyrosine (a neutral amino acid) to be soluble at ph 8? Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Below ph = 1.7 (pka1) h3a2+ is the most abundant specie. Draw the predominate form of histidine at physiological ph. Draw arginine in its predominant form at ph = 10.0. Draw the predominant form of each amino acid at ph 7.0.
Draw The Predominant Form Of Histidine At Ph 0 at Drawing
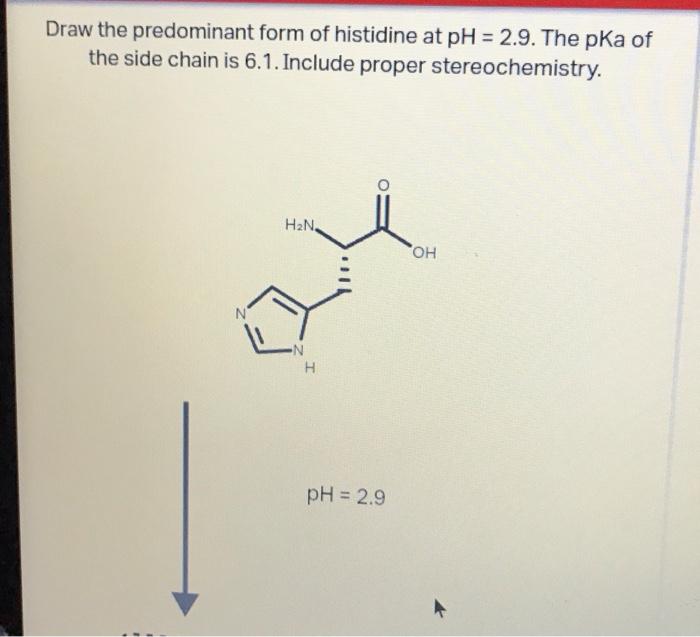
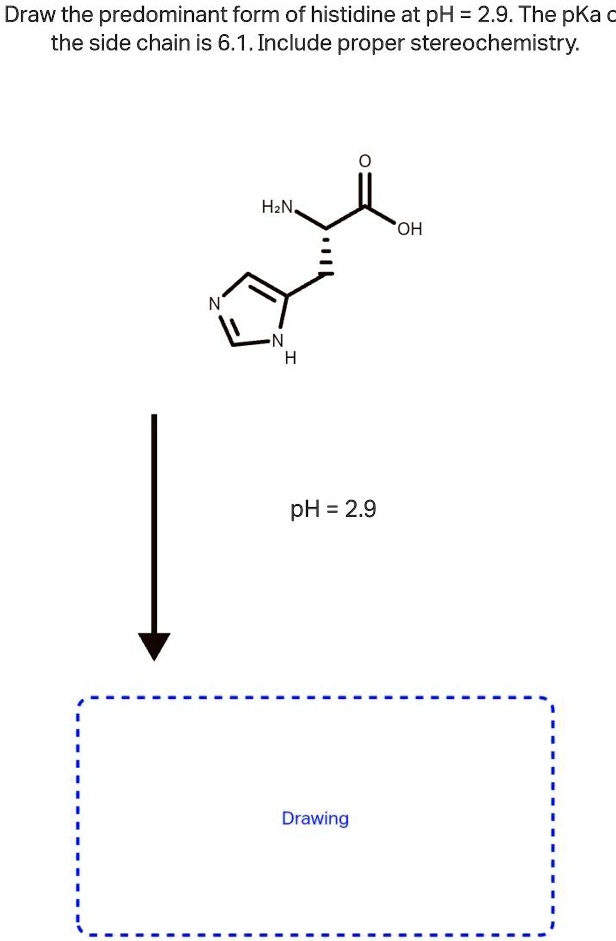
Draw the predominant form of each amino acid at ph 7.0. Write the approximate pk_aof each group next to its position in your structure. Draw the predominant form of histidine at ph=2.9. Web there are 3 steps to solve this one. Identify the amino acid with the lowest pl (try to solve this problem by inspecting their structures, rather than.
Histidine chemical formula. Histidine chemical molecular structure
Web ph = 0 ph = 7 ph = 11 ho2. This problem has been solved! Write the approximate pk_aof each group next to its position in your structure. At ph = 1.7 there is a equal amount of h3a2 + and h2a+. As the pka is very close to the ph, only small changes in the local environment can.
Solved Draw the structure of histidine at the following
This problem has been solved! Write the approximate pk_aof each group next to its position in your structure. Draw arginine in its predominant form at ph = 2.0. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbar active by default. Draw the structure of the major form of phenylalanine at.
Histidine Titration Curve
At ph = 6.02 there is equal amounts of h2a+ and ha. This is close to physiological ph (i.e. At ph 2 at ph 7.8 at ph 10 would you expect the amino acid tyrosine (a neutral amino acid) to be soluble at ph 8? Draw the predominant form of histidine at ph=0. Draw arginine in its predominant form at.
[Solved] 1. Draw the structure of histidine that predominates at a pH
To find the pl (isoelectric point), we need to find the ph at which the net charge of histidine is 0. This is because the ph scale measures the concentration of hydrogen ions in a solution, and at low ph, there is an abundance of h⁺. Here’s the best way to solve it. (a) in ph 5 b, in ph.
draw the predominant form of histidine at mathrmph29 the mathrmpka of
Arginine and histidine are basic amino acids. This is because the ph scale measures the concentration of hydrogen ions in a solution, and at low ph, there is an abundance of h⁺. Pka 3.86 nh2 o ho o oh glutamic acid pka 4.25 histidine nh2 o ho n+ n h h pka 6.04 nh2 o ho sh cysteine pka 8.35.
SOLVED a)Draw a Titration curve of histidine from pH 114. b) Draw
Best matched videos solved by our top educators. Zwitterions problem 8 draw the. (a) in ph 5 b, in ph 10 c, in ph 10.9 d, what is the net charge of histidine at the ph 10. This occurs between pka2 and pka3, so we can calculate the pl as the average of these two values: Calculate the ratio of.
Solved 18. Draw the structure of histidine at the pH values
As the pka is very close to the ph, only small changes in the local environment can change the protonation state of the amino acid. Web the conjugate acid (protonated form) of the imidazole side chain in histidine has a pk a of approximately 6.0. Draw the predominant form of histidine at ph=2.9. Glycine, aspartic acid, or lysine. This problem.
SOLVEDDraw the most predominant form of histidine at its isoelectric
Below ph = 1.7 (pka1) h3a2+ is the most abundant specie. Glycine, aspartic acid, or lysine. This is because the ph scale measures the concentration of hydrogen ions in a solution, and at low ph, there is an abundance of h⁺. Draw arginine in its predominant form at ph = 10.0. This occurs between pka2 and pka3, so we can.
SOLVED Draw the predominant form of histidine at pH=2.9. The pKa of
Draw arginine in its predominant form at ph = 2.0. To find the pl (isoelectric point), we need to find the ph at which the net charge of histidine is 0. This problem has been solved! The pka of the side chain is 6.1. Draw the predominant form of a given amino acid in a solution of known ph, given.
Arginine And Histidine Are Basic Amino Acids.
Draw the structure of the major form of phenylalanine at phvalues of 1, 5.5, and 11.(b) the isoelectric point of histidine is ph 7.6. Write the approximate pk_aof each group next to its position in your structure. Below ph = 1.7 (pka1) h3a2+ is the most abundant specie. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis.
Draw Each Structure And Analyze.
At low ph, such as ph=0, histidine exists primarily in its protonated form as h⁺. Video answers to similar questions. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbar active by default. Draw the structures of the major forms of histidine at ph values of 1, 4,7.6, and 11.
The Pl Of Histidine Is $\Boldsymbol {7.6}$.
The predominant form of histidine at ph=0 is: Draw arginine in its predominant form at ph = 2.0. At ph 2 at ph 7.8 at ph 10 would you expect the amino acid tyrosine (a neutral amino acid) to be soluble at ph 8? You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
As The Pka Is Very Close To The Ph, Only Small Changes In The Local Environment Can Change The Protonation State Of The Amino Acid.
Describe, briefly, how a mixture of amino. Draw histidine in its predominant form at ph = 2.0. Web the conjugate acid (protonated form) of the imidazole side chain in histidine has a pk a of approximately 6.0. Draw the predominant form of histidine at ph=0.