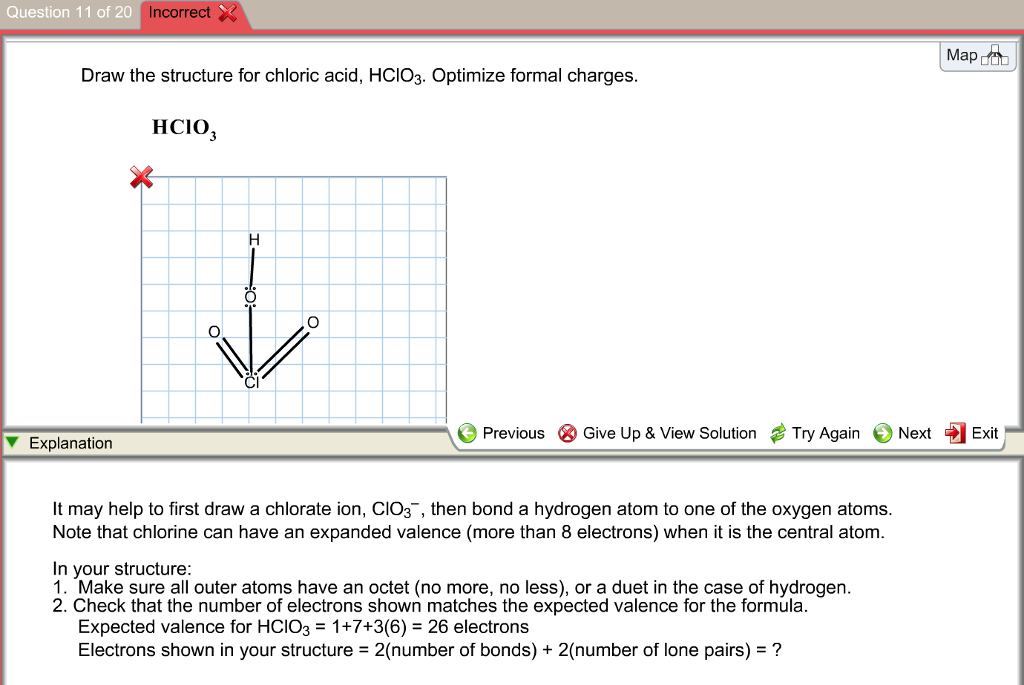
Draw The Structure For Chloric Acid Hclo3 Optimize Formal Charges
Draw The Structure For Chloric Acid Hclo3 Optimize Formal Charges - No one rated this answer yet — why not be the first? The best and most stable lewis structure of hclo3 with minimized formal charges is. When we have an h (or h2) in front of a polyatomic molecule (like co3,. Lab techniques and procedures 1h 38m. Draw the structure for chloric acid, hclo3. The given molecular formula is hclo3. Chlorine is able to have an expanded octet. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Because of this reason, the above obtained lewis structure of hclo3 is not stable. This problem has been solved!
#4 calculate formal charge and check stability (if octet is already completed on central atom) #5 convert lone pair and calculate formal charge again (if formal charges are not closer to zero) The given molecular formula is hclo3. Chlorine is able to have an expanded octet. Web what is the lewis structure for hclo? Everything else is 0, though. Treichel, john townsend, david treichel. Since we want our formal charges to be as close to 0 as possible, we're going to need to do something to minimize the charges. 83% (6 ratings) share share. There are 2 steps to solve this one. When determining the formal charge of an atom in a molecule, we assign electrons to atoms based on their electronegativity and bonding patterns.
5.4k views 3 years ago. Draw the structure for chloric acid, hcio,. Everything else is 0, though. Web what is the lewis structure for hclo? The formal charge of chlorine in chloric acid (hclo) is +3. Draw the structure for chloric acid, hclo3. The given molecular formula is hclo3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The formal charge of chlorine (cl) in chloric acid (hclo3) can be calculated by subtracting the number of valence electrons in the free chlorine atom from the number of valence electrons assigned to chlorine in the compound. This youtube video should also help:
Solved Draw The Structure For Chloric Acid, HClO_3. Optim...
Click on a star to rate it! Web in summary, the formal charge on chlorine in hclo3 is +2 when all the bonds on chlorine are single bonds, as opposed to +5 or +7 when oxygen and chlorine form double bonds. #4 calculate formal charge and check stability (if octet is already completed on central atom) #5 convert lone pair.
Solved 3.1 Draw a Lewis structure for chloric acid, HClO3.
In many sites, i see that they put o o in the middle, but our teacher said that we should put o o near the extremity. So we have to minimize these charges by shifting the electron pairs towards the chlorine atom. Formal charge = [# of valence. Select draw rings more // iii h 0 ci. This should be.
SOLVED Draw the structure for chloric acid HClO3.
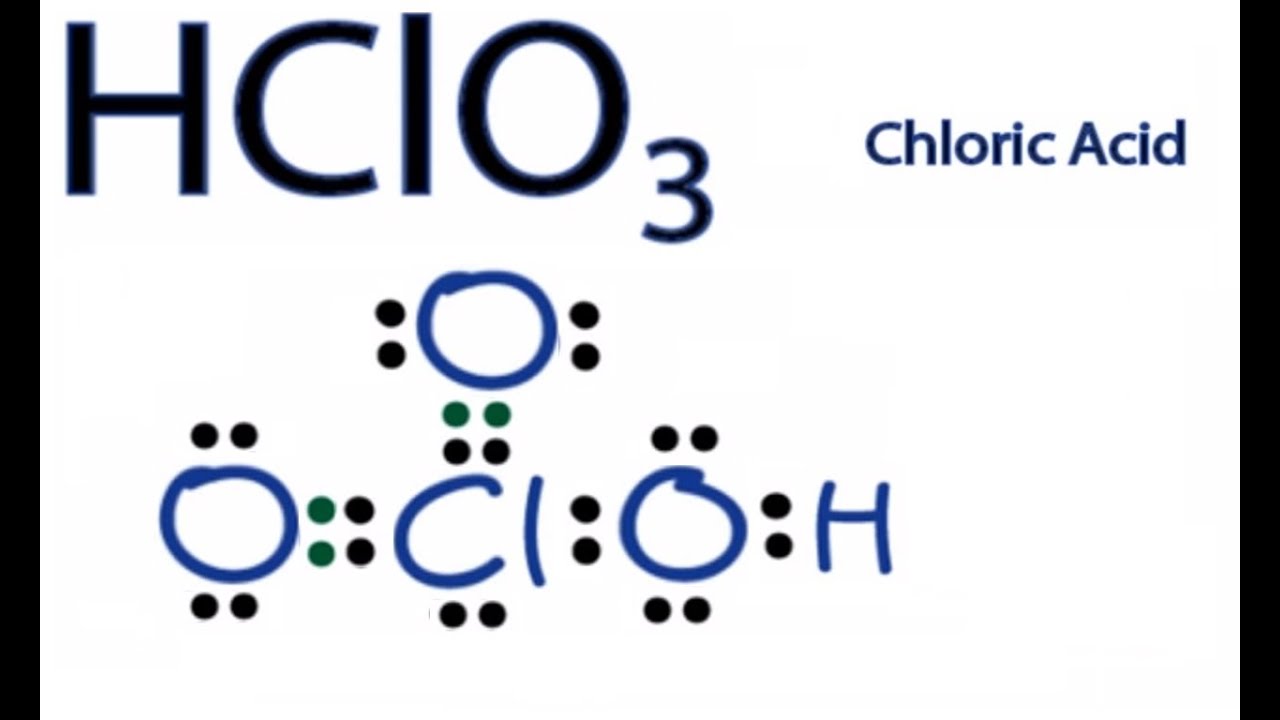
No one rated this answer yet — why not be the first? There are 2 steps to solve this one. #1 first draw a rough sketch. Web to minimize the formal charges, take one of the lone pairs from each oxygen and convert the single bond into a double bond. Which is the best way?
SOLVED For the molecule HClO3 (chloric acid) (1) Write all possible
In order to calculate the formal charges for hclo3 we'll use the equation: Web in summary, the formal charge on chlorine in hclo3 is +2 when all the bonds on chlorine are single bonds, as opposed to +5 or +7 when oxygen and chlorine form double bonds. Lab techniques and procedures 1h 38m. Draw the structure for chloric acid, hclo3..
Hclo3
Everything else is 0, though. The formal charge of chlorine (cl) in chloric acid (hclo3) can be calculated by subtracting the number of valence electrons in the free chlorine atom from the number of valence electrons assigned to chlorine in the compound. #4 calculate formal charge and check stability (if octet is already completed on central atom) #5 convert lone.
HClO3 Lewis Structure How to Draw the Lewis Structure for HClO3 YouTube
So we have to minimize these charges by shifting the electron pairs towards the chlorine atom. This youtube video should also help: Atoms & elements 4h 15m. Asked 9 years, 3 months ago. Draw the structure for chloric acid, hclo3.
(Solved) Question 1 Chloric Acid (HClO3) Is A Powerful Oxidizer And
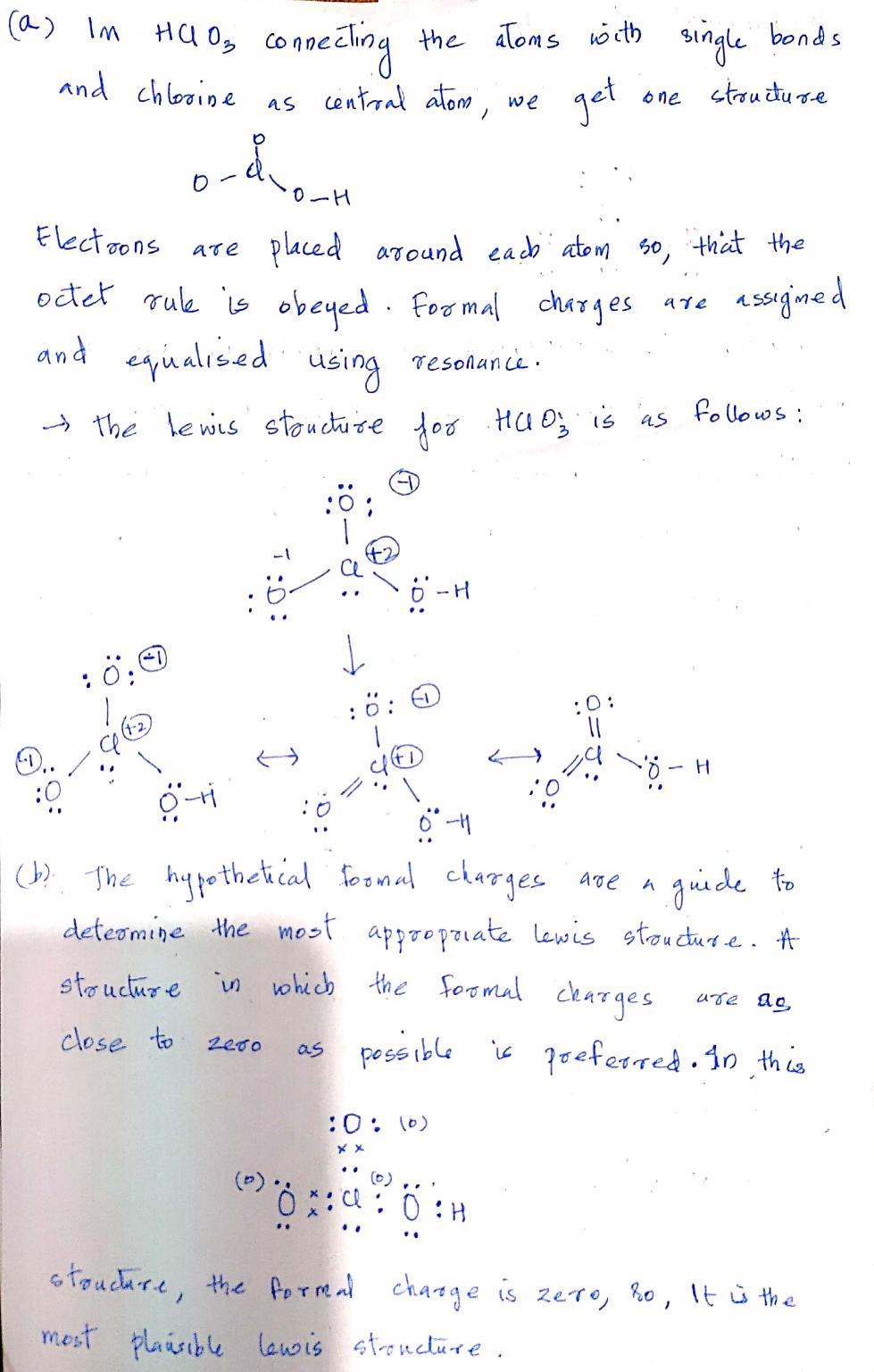
The best and most stable lewis structure of hclo3 with minimized formal charges is. Web in summary, the formal charge on chlorine in hclo3 is +2 when all the bonds on chlorine are single bonds, as opposed to +5 or +7 when oxygen and chlorine form double bonds. 5.4k views 3 years ago. Select draw rings more // iii h.
10. Draw the structure for Chloric acid. What > th… SolvedLib
Draw the structure for chloric acid, hcio,. Table \(\pageindex{1}\) lists the various oxyacids of chlorine. This youtube video should also help: The best and most stable lewis structure of hclo3 with minimized formal charges is. The relative strengths increase with the number of oxygen atoms since the more there are, the greater is the extent to which the negative charge.
How to Calculate the Formal Charges for HClO3 (Choric acid) YouTube
Intro to general chemistry 3h 51m. The formal charge of chlorine (cl) in chloric acid (hclo3) can be calculated by subtracting the number of valence electrons in the free chlorine atom from the number of valence electrons assigned to chlorine in the compound. Create an account to view solutions. In this structure, the central atom is carbon (c), bonded to.
Chloric acid. Molecular model of the strong acid and oxidizing agent
There are 2 steps to solve this one. #4 calculate formal charge and check stability (if octet is already completed on central atom) #5 convert lone pair and calculate formal charge again (if formal charges are not closer to zero) #3 calculate and mark formal charges on the atoms, if required. #2 mark lone pairs on the atoms. You'll get.
Chlorine Is Able To Have An Expanded Octet.
The formal charge of chlorine (cl) in chloric acid (hclo3) can be calculated by subtracting the number of valence electrons in the free chlorine atom from the number of valence electrons assigned to chlorine in the compound. Click on a star to rate it! #3 calculate and mark formal charges on the atoms, if required. So we have to minimize these charges by shifting the electron pairs towards the chlorine atom.
#2 Mark Lone Pairs On The Atoms.
Since we want our formal charges to be as close to 0 as possible, we're going to need to do something to minimize the charges. When we have an h (or h2) in front of a polyatomic molecule (like co3,. Which is the best way? In this structure, the central atom is carbon (c), bonded to three oxygen atoms (o).
#4 Calculate Formal Charge And Check Stability (If Octet Is Already Completed On Central Atom) #5 Convert Lone Pair And Calculate Formal Charge Again (If Formal Charges Are Not Closer To Zero)
2 posts • page 1 of 1. Web the structure of chloric acid, hclo3, consists of a chlorine atom bonded to three oxygen atoms, with two of the oxygen atoms carrying a single negative charge. Web to minimize the formal charges, take one of the lone pairs from each oxygen and convert the single bond into a double bond. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Formal Charge = [# Of Valence.
The formal charges of the atoms are optimized to achieve a neutral overall charge. The molecular name for the given compound is chloric acid. Web interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university courses and advanced school chemistry hosted by university of liverpool. The best and most stable lewis structure of hclo3 with minimized formal charges is.