Draw The Structure Of Cyclobutane
Draw The Structure Of Cyclobutane - Web draw structures of the following derivatives. What sets cyclobutane apart from other cycloalkanes is the. Stable cycloalkanes cannot be formed with carbon chains of just any length. These cycloalkanes do not have the same molecular formula, so the heat of combustion per each ch 2 unit present in each molecule is calculated (the fourth column) to provide a useful comparison. Some helocs offer a discounted teaser rate for a period before switching to a higher fully indexed rate later on. The cyclobutane molecule contains a total of 12 bond (s). The name of this molecule is cyclobutylcyclopentane. Web in a line drawing, this butterfly shape is usually shown from the side, with the near edges drawn using darker lines. Because it contains more carbons, the cyclopentane ring will be named as the parent chain. It is composed of four carbon atoms (c) and eight hydrogen atoms (h), forming a cyclic structure.
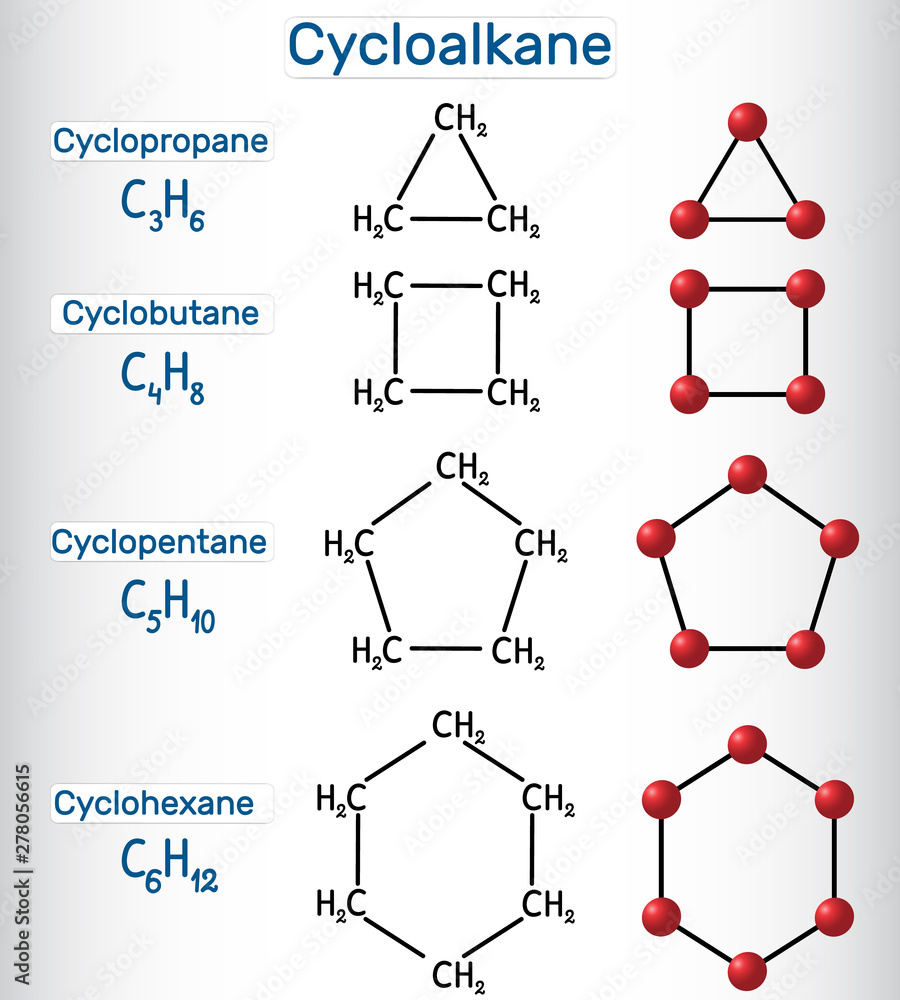
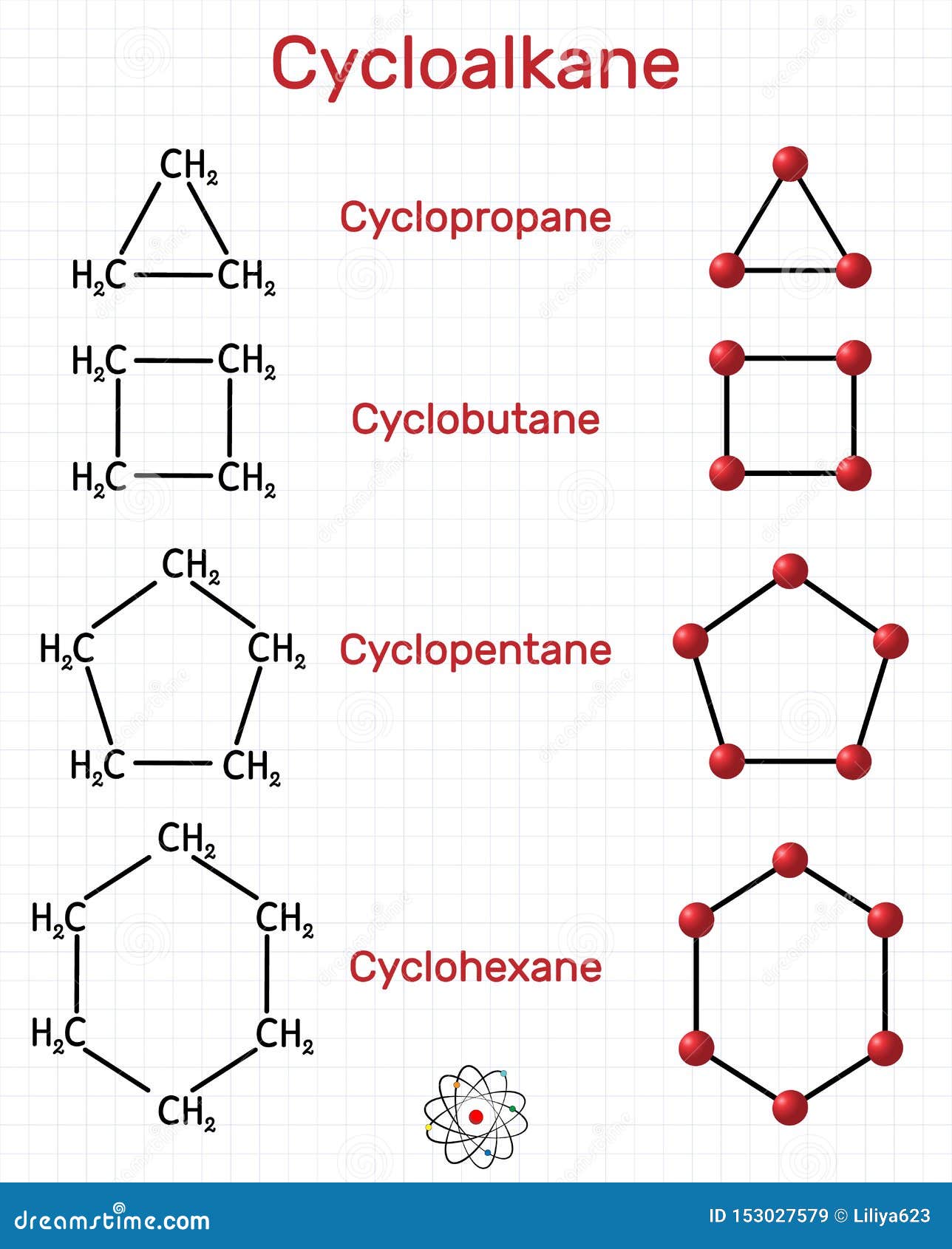
Generally, cycloalkanes are cyclic molecules consisted of purely carbon and hydrogen atoms. The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. Stable cycloalkanes cannot be formed with carbon chains of just any length. Web cycloalkanes are alkanes that are in the form of a ring; Web cyclohexane, for example, has a ring structure that looks like this: Because it contains more carbons, the cyclopentane ring will be named as the parent chain. Web cyclobutane, 4th member of homologous order of cycloalkenes, is a molecule with the molecular formula of c x 4 h x 8 \ce{c4h8} c x 4 h x 8. According to a classification scheme (2), this estimated koc value suggests that cyclobutane is expected to have very high mobility in soil (src). Web a) 1), 2) structures of the reaction are given below in the attachment. Web cyclobutane, a member of the cycloalkane series, is chemically represented as c 4 h 8.
Recall that in alkanes, carbon adopts the sp 3 tetrahedral geometry in which the angles between bonds are 109.5°. From the data, cyclopropane and cyclobutane have. According to a classification scheme (2), this estimated koc value suggests that cyclobutane is expected to have very high mobility in soil (src). 100% (3 ratings) share share. For example, the saturated hydrocarbon with 4 numbers of carbon atoms is named. Now describe the structure you have drawn: The number of carbon atoms present in the compound decides the structure of cycloalkane. Use this link for bookmarking this species for future reference. Stable cycloalkanes cannot be formed with carbon chains of just any length. A chemist would say that the ring has one degree of unsaturation with respect to the parent alkane.
[Solved] Draw the structure of cyclobutane. Draw the molecule on the
Stable cycloalkanes cannot be formed with carbon chains of just any length. The name of this molecule is cyclobutylcyclopentane. Web cyclobutane is a cycloalkane and organic compound with the formula (ch 2) 4. Their empirical formula can be written as: For some cycloalkanes to form, the angle between bonds must.
Cyclobutane Chemical Structure Vector Design Illustration Stock Vector
Generally, cycloalkanes are cyclic molecules consisted of purely carbon and hydrogen atoms. What sets cyclobutane apart from other cycloalkanes is the. In many cases, it's wise to avoid these and opt for. A chemist would say that the ring has one degree of unsaturation with respect to the parent alkane. Web cyclobutane as a result, the total strain for the.
Cyclobutane Structural Formula
Torsional strain is still present, but the neighbouring bonds are not exactly eclipsed in the butterfly. Web some common examples of cycloalkanes are the cyclopentane, cyclobutane, cyclohexane, and cycloheptane, cyclooctane, etc as shown below in the image. Use this link for bookmarking this species for future reference. Web cyclohexane, for example, has a ring structure that looks like this: B).
Cyclobutane C4H8 Organic Compound Molecular Structure Stock Vector
Web a) 1), 2) structures of the reaction are given below in the attachment. The name of this molecule is cyclobutylcyclopentane. Because it contains more carbons, the cyclopentane ring will be named as the parent chain. With bond angles of 88 rather than 109 degrees, cyclobutane has a lot of ring strain, but less than in cyclopropane. Use this link.
Cyclobutane Structural Formula
(vi) the methyl hemiacetal of formaldehyde. The cyclohexane molecule is constantly changing, with the atom on the left, which is currently pointing down, flipping up, and the atom on the right flipping down. Web cycloalkanes are alkanes that are in the form of a ring; 100% (3 ratings) share share. Images of the chemical structure of cyclobutane are.
Structural Formula for Cyclobutane (and molecular formula) YouTube
The cyclohexane molecule is constantly changing, with the atom on the left, which is currently pointing down, flipping up, and the atom on the right flipping down. Web draw structures of the following derivatives. The number of carbon atoms present in the compound decides the structure of cycloalkane. Web using a structure estimation method based on molecular connectivity indices (1),.
Draw the structural formula of cyclobutane Science Carbon and its
Un 2601 permanent link for this species. Stable cycloalkanes cannot be formed with carbon chains of just any length. For example, the saturated hydrocarbon with 4 numbers of carbon atoms is named. As such, cyclobutane is unstable above about 500 °c. Their empirical formula can be written as:
3D image of cyclobutane skeletal formula molecular chemical structure
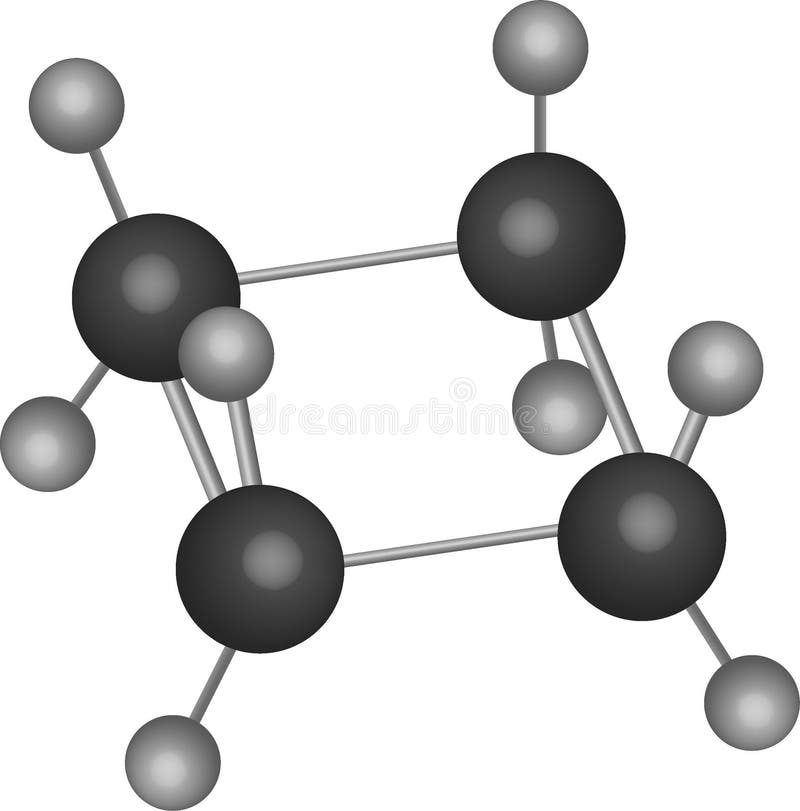
Cyclobutane has 2 hydrogen atoms less than does butane, c 4 h 10. C x n h x 2 n \ce{c_nh_{2n}} c x n h x 2 n cyclobutane can be. Web draw the structure of cyclobutane. Use this link for bookmarking this species for future reference. Web in a line drawing, this butterfly shape is usually shown from the.
Fototapeta Chemical formula and molecule model cyclopropane C3H6
Web using a structure estimation method based on molecular connectivity indices (1), the koc for cyclobutane can be estimated to be about 49 (src). With bond angles of 88 rather than 109 degrees, cyclobutane has a lot of ring strain, but less than in cyclopropane. C x n h x 2 n \ce{c_nh_{2n}} c x n h x 2 n.
Cyclobutane C4H8 Organic Compound Molecular Structure Vector
The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. These cycloalkanes do not have the same molecular formula, so the heat of combustion per each ch 2 unit present in each molecule is calculated (the fourth column) to provide a useful comparison. Web cyclobutane as a.
This Structure Is Also Available As A 2D Mol File Or As A Computed 3D Sd File The 3D Structure May Be Viewed Using Java Or Javascript.
As such, cyclobutane is unstable above about 500 °c. These cycloalkanes do not have the same molecular formula, so the heat of combustion per each ch 2 unit present in each molecule is calculated (the fourth column) to provide a useful comparison. Images of the chemical structure of cyclobutane are. It is composed of four carbon atoms (c) and eight hydrogen atoms (h), forming a cyclic structure.
What Sets Cyclobutane Apart From Other Cycloalkanes Is The.
(iv) the semicarbazone of cyclobutanone. This is known as the chair form of cyclohexane from its shape, which vaguely resembles a chair. Cyclobutane has 2 hydrogen atoms less than does butane, c 4 h 10. The carbon atoms are joined in a ring, each bonded to two other carbons and two hydrogen atoms.
Recall That In Alkanes, Carbon Adopts The Sp 3 Tetrahedral Geometry In Which The Angles Between Bonds Are 109.5°.
Their empirical formula can be written as: Now describe the structure you have drawn: Web draw structures of the following derivatives. There are two different cycloalkanes in this molecule.
Web Using A Structure Estimation Method Based On Molecular Connectivity Indices (1), The Koc For Cyclobutane Can Be Estimated To Be About 49 (Src).
Use this link for bookmarking this species for future reference. There are 2 steps to solve this one. The number of carbon atoms present in the compound decides the structure of cycloalkane. Web cyclobutane as a result, the total strain for the two compounds is nearly the same—110 kj/mol (26.4 kcal/mol) for cyclobutane versus 115 kj/mol (27.5 kcal/mol) for cyclopropane.