Drawing Covalent Compounds
Drawing Covalent Compounds - Web in this video, we will go through how to draw lewis structures for covalent compound in five easy steps using carbon dioxide, co2, as an example. Which of the following correctly completes the lewis. Determine the total number of valence electrons in the molecule or ion. Learn to draw lewis structures for covalent molecules containing double and triple bonds. Web drawing lewis structures for molecules with one central atom: Lewis structures of covalent compounds and ions. Web how to draw covalent bonding molecules. Draw lewis structures depicting the bonding in simple molecules. Drawing lewis structures for bf3, pf3 and brf3; Each line represents two electrons that are being shared.
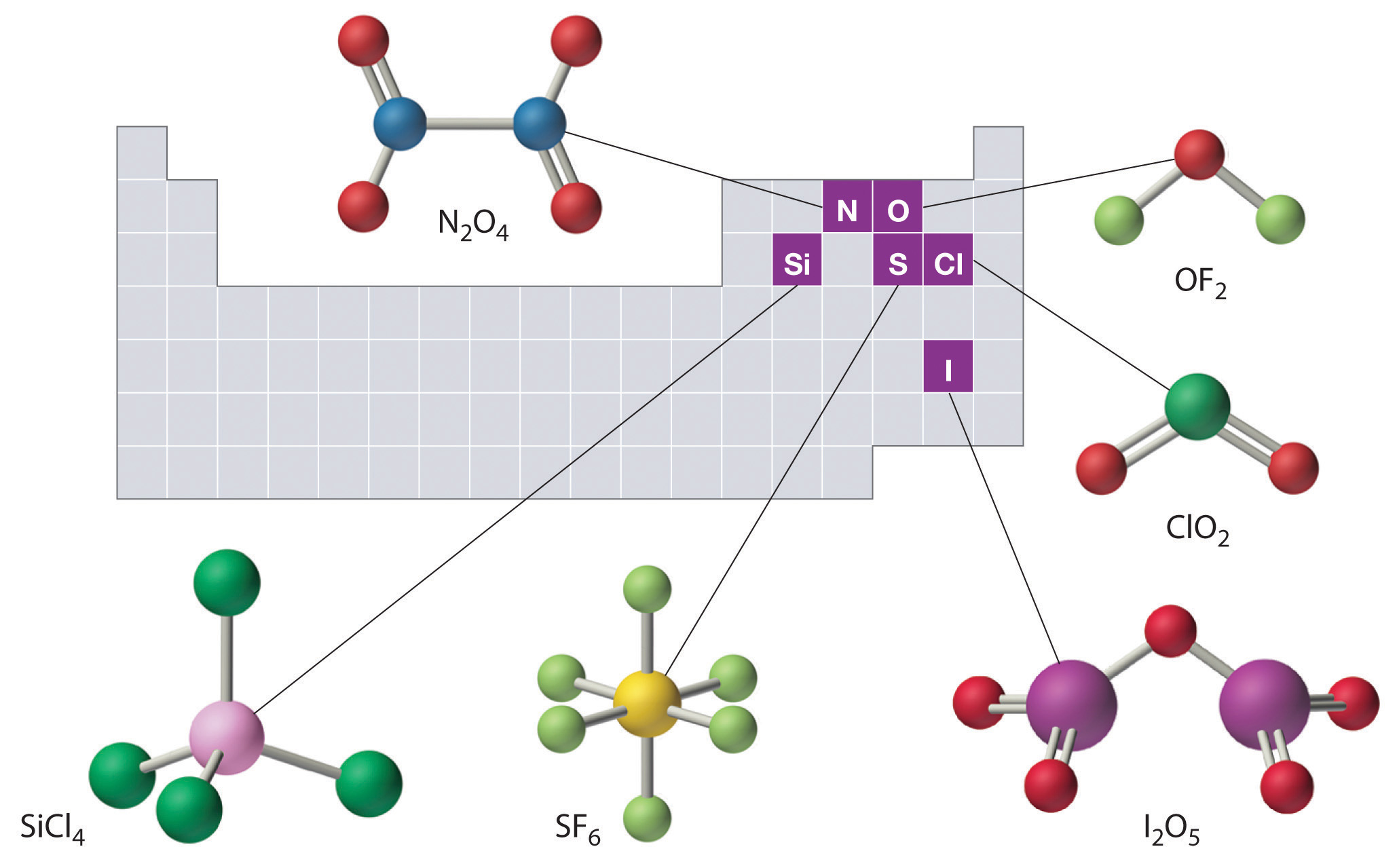
Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, In this video you’ll learn how to draw lewis dot structures for covalent compounds. For example, consider ccl4 and nf3 as drawn below: Determine the total number of valence electrons in the molecule or ion. What is an example of a lewis structures practice problem? Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. Web covalent bonds are produced when unpaired electrons found within two atoms interact to form a shared pair of electrons. Draw lewis structures for covalent compounds. Each line represents two electrons that are being shared.
Web relates to covalent bonding. How do you draw the lewis structure for ionic compounds? The video covers the basic lewis structures you'll see in an introductory chemistry. Web to recognize molecules that are likely to have multiple covalent bonds. Draw lewis structures for covalent compounds. Web in this video, we will go through how to draw lewis structures for covalent compound in five easy steps using carbon dioxide, co2, as an example. Determine the total number of valence electrons in the molecule or ion. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. Web how to draw covalent bonding molecules. Determine the total number of valence electrons in the molecule or ion.
How To Draw Covalent Bonds
Which of the following correctly completes the lewis. Web relates to covalent bonding. Draw lewis structures depicting the bonding in simple molecules. Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
3.6 Naming Covalent Compounds Chemistry LibreTexts
The skeletal structure of ethanethiol is shown below. Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. Lewis structures of covalent compounds and ions. Notice that the atoms share electrons so that they all have 8 electrons. The compound is often added to otherwise odorless fuels such as natural gas to.
Covalent Compounds
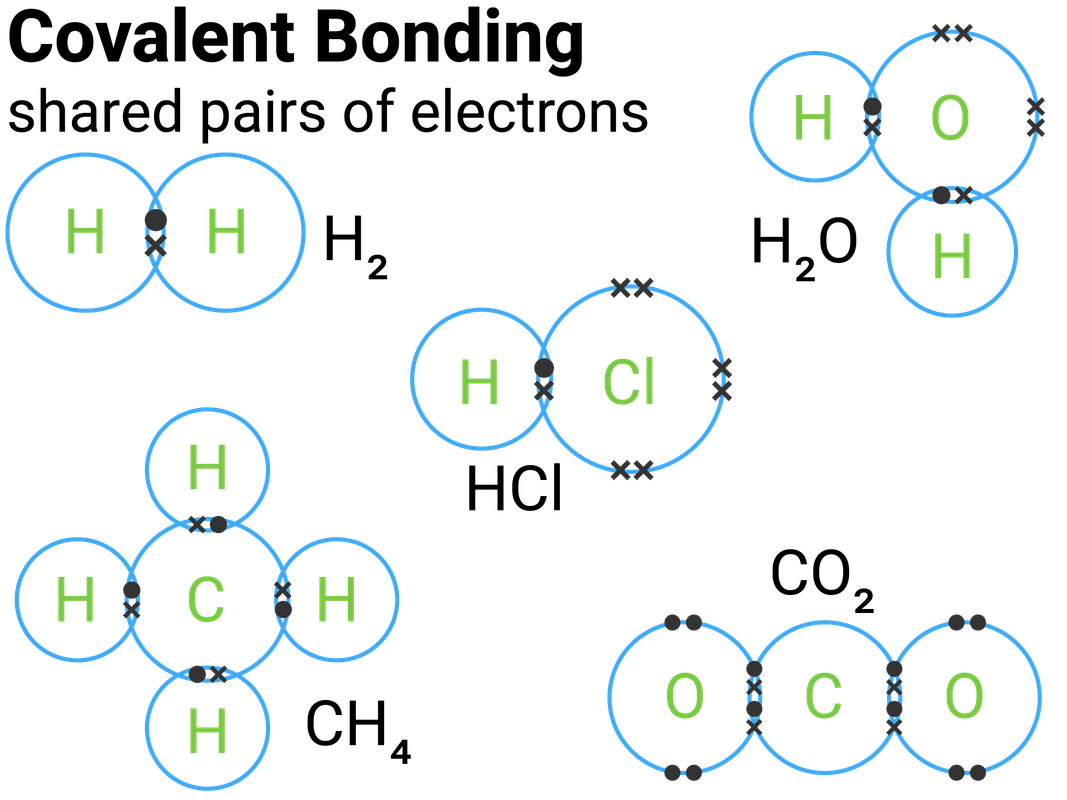
Web learn to draw lewis structures for covalent molecules containing only single bonds. Draw lewis structures depicting the bonding in simple molecules. For covalent bonding, we often want to draw how the atoms share electrons in the molecule. Remember that lewis dot structures. 0:08 introduction 0:39 h2 1:25 hcl.
How to Draw a Lewis Structure
Drawing lewis structures for bf3, pf3 and brf3; Notice that the atoms share electrons so that they all have 8 electrons. Examples for drawing lewis structures for covalent bonds. Learn to draw lewis structures for covalent molecules containing double and triple bonds. Determine the total number of valence electrons in the molecule or ion.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Each line represents two electrons that are being shared. Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. The video covers the basic lewis structures you'll see in an introductory chemistry. Examples for drawing lewis.
How to Draw Lewis Dot Structure of Covalent Compounds Chemical
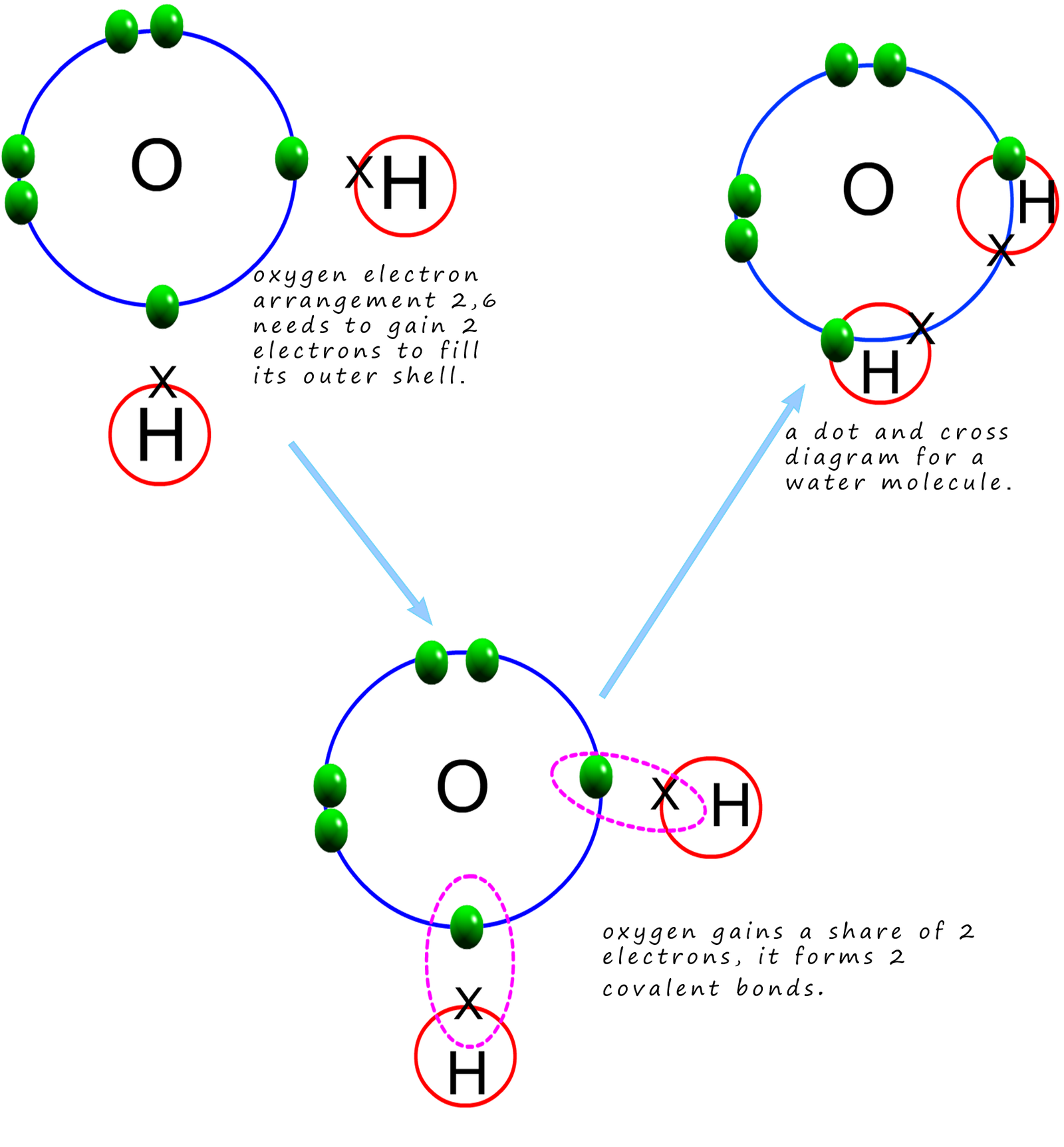
Web illustrate covalent bond formation with lewis electron dot diagrams. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Which of the following correctly completes the lewis. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species.
Covalent bonding
The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Drawing lewis dot structures for polyatomic ions. How do you draw the lewis structure for ionic compounds? Web how to.
Examples of Covalent Bonds and Compounds
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The following procedure can be used to draw lewis structure for simple molecules. Web draw lewis structures for covalent compounds. The only thing smaller than atoms is their subatomic particles; Using formal charges to determine how many bonds to make, a different perspective.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Learn to use vsepr theory to determine molecular shape of covalent molecules. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Drawing lewis diagrams for covalent compounds. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons.
Covalent Compounds Examples and Properties
Web illustrate covalent bond formation with lewis electron dot diagrams. Web learn to draw lewis structures for covalent molecules containing only single bonds. Determine the total number of valence electrons in the molecule or ion. Web how to draw a lewis structure. Using formal charges to determine how many bonds to make, a different perspective.
Examples For Drawing Lewis Structures For Covalent Bonds.
Web drawing lewis structures for molecules with one central atom: 0:08 introduction 0:39 h2 1:25 hcl. What is an example of a lewis structures practice problem? Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected.
How Do You Draw The Lewis Structure For Ionic Compounds?
Learn to draw lewis structures for covalent molecules containing double and triple bonds. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. For example, consider ccl4 and nf3 as drawn below: The skeletal structure of ethanethiol is shown below.
Lewis Structure, Also Known As Lewis Dot Structure Or Electron Dot Structure, Is A Simple And Straightforward Way Of Representing The Outermost Electron Shell In A Chemical Species Like An Atom, Ion, Or Molecule.
Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the first shell. The example is for the nitrate ion. Determine the total number of valence electrons in the molecule or ion. Learn to use vsepr theory to determine molecular shape of covalent molecules.
For Very Simple Molecules And Molecular Ions, We Can Write The Lewis Structures By Merely Pairing Up The Unpaired Electrons On The Constituent Atoms.
Web how to draw covalent bonding molecules. The video covers the basic lewis structures you'll see in an introductory chemistry. Web draw lewis structures for covalent compounds. Web in this video, we will go through how to draw lewis structures for covalent compound in five easy steps using carbon dioxide, co2, as an example.



/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)

