Drawing Lewis Structures For Simple Organic Compounds
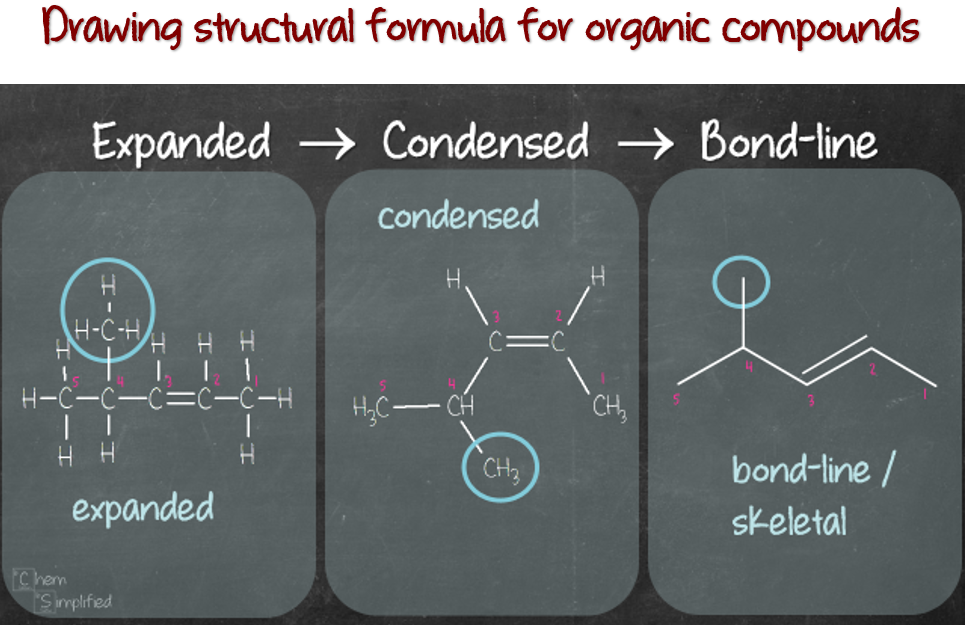
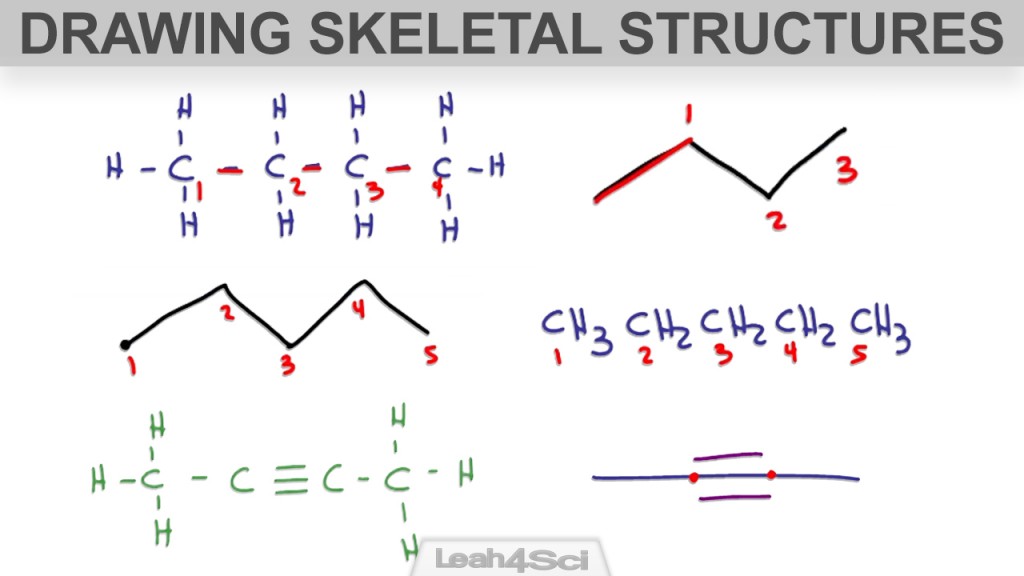
Drawing Lewis Structures For Simple Organic Compounds - For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Molecules can be represented using lewis structures, which show how electrons are arranged around the atoms in a molecule as bonded pairs of electrons (bonds) and lone pairs of electrons. Web heather mathis view bio. What are some examples of lewis structures? Web lewis structures for simple organic compounds. What is an example of a lewis structures practice problem? High school chemistry skills practice. Web more commonly, organic and biological chemists use an abbreviated drawing convention called line structures. Answers to chapter 1 practice questions. Carbon atoms are depicted not by a capital c, but by a ‘corner’ between two bonds or a free end of a bond.
Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Sum the valence electrons from all the atoms. Web heather mathis view bio. Web draw out a correct lewis structure for the following compounds. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. Steps for drawing lewis structures for simple organic compounds. Carbon atoms are depicted not by a capital c, but by a ‘corner’ between two bonds or a free end of a bond. Use the following procedure as a guide to creating lewis structures. Another simple and general procedure to draw lewis structures has. Molecules can be represented using lewis structures, which show how electrons are arranged around the atoms in a molecule as bonded pairs of electrons (bonds) and lone pairs of electrons.
Web draw the lewis structure of the n 2 molecule. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Shared pairs of electrons are drawn as lines between atoms, while. The example is for the nitrate ion. Two chlorines are bonded to a sulfur. 31k views 1 year ago organic chemistry videos. Carbon atoms are depicted not by a capital c, but by a ‘corner’ between two bonds or a free end of a bond. Sum the valence electrons from all the atoms. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Web by james ashenhurst.
Organic Chemistry 101 Drawing the structures ChemSimplified
For more complicated polyatomic molecules and ions, the lewis structures cannot be obtained by simply combining lewis symbols. Web drawing lewis structures. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. Steps for drawing lewis structures for simple organic compounds. Web draw the lewis structure.
2.9 Some Simple Organic Compounds Chemistry LibreTexts
Two chlorines are bonded to a sulfur. What is an example of a lewis structures practice problem? Web drawing lewis structures. 1.2.2 lewis structures of polyatomic molecules or ions. What is the lewis structure for so2?
How to Draw a Lewis Structure
31k views 1 year ago organic chemistry videos. Web common bonding patterns in organic structures. Answers to chapter 1 practice questions. Web here are the steps to draw a lewis structure. What are some examples of lewis structures?
Organic Chemistry Drawing Structures
Answers to chapter 1 practice questions. There are essentially three uses for lewis structures. Web drawing lewis structures for simple organic compounds practice | chemistry practice problems | study.com. Steps for drawing lewis structures for simple organic compounds. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone.
9.1k Drawing Lewis structures for simple organic compounds YouTube
High school chemistry skills practice. Web this organic chemistry video tutorial shows you how to draw lewis structures, bond line structures and skeletal structures including condensed structural for. Web common bonding patterns in organic structures. For more complicated polyatomic molecules and ions, the lewis structures cannot be obtained by simply combining lewis symbols. Different compounds having the same molecular formula.
Drawing Skeletal Structures of Organic Compounds Tutorial Video
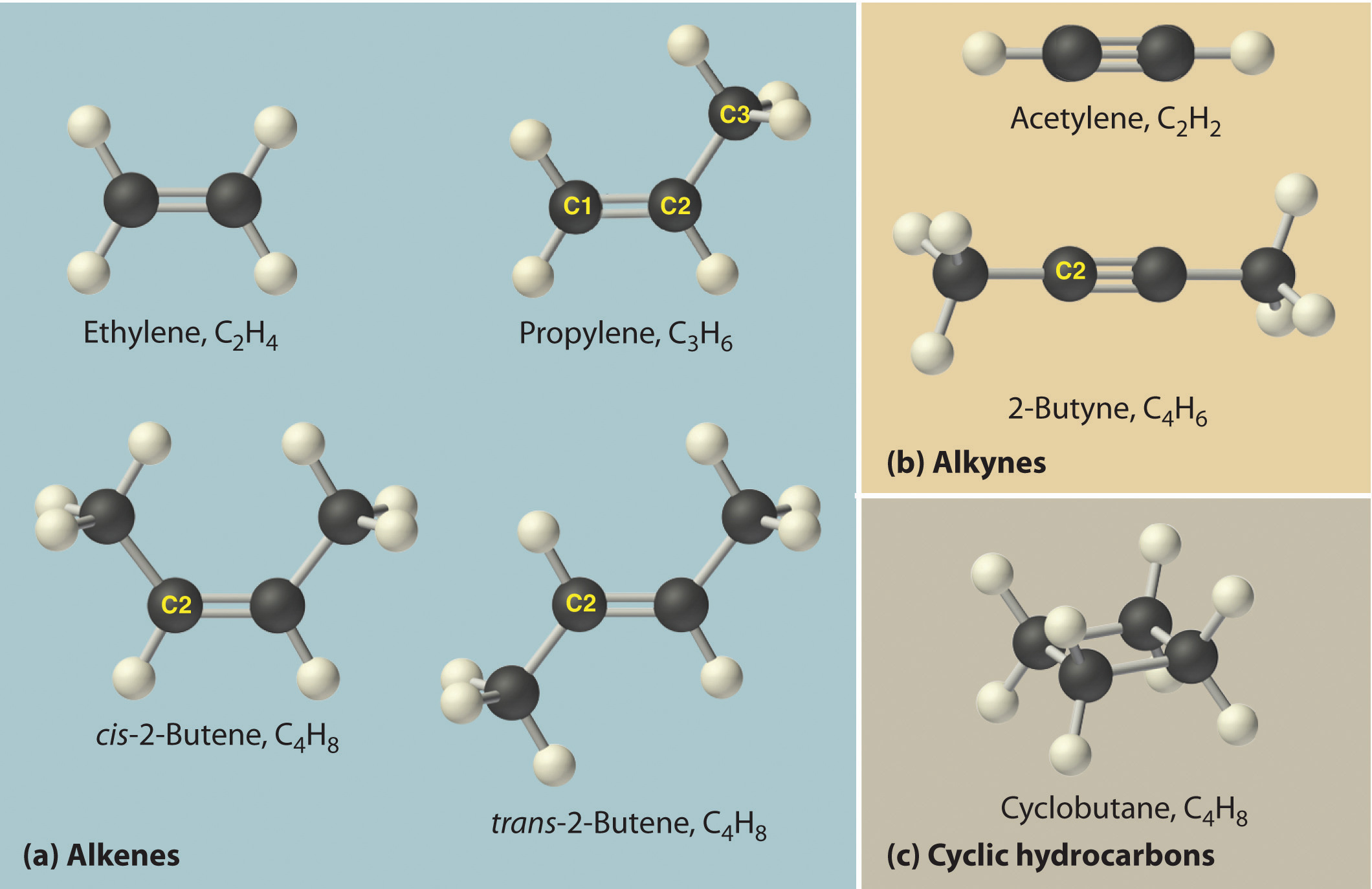
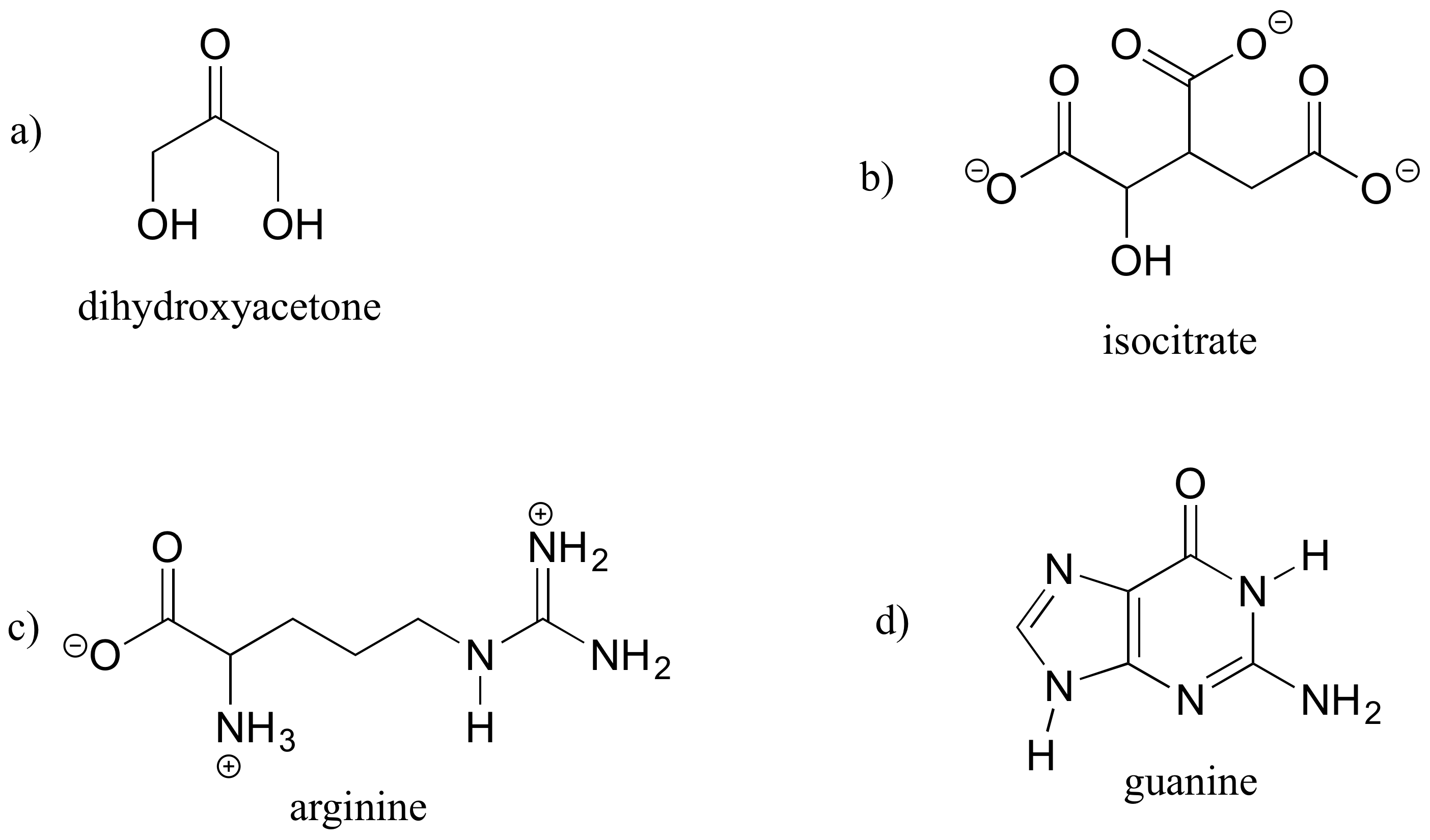
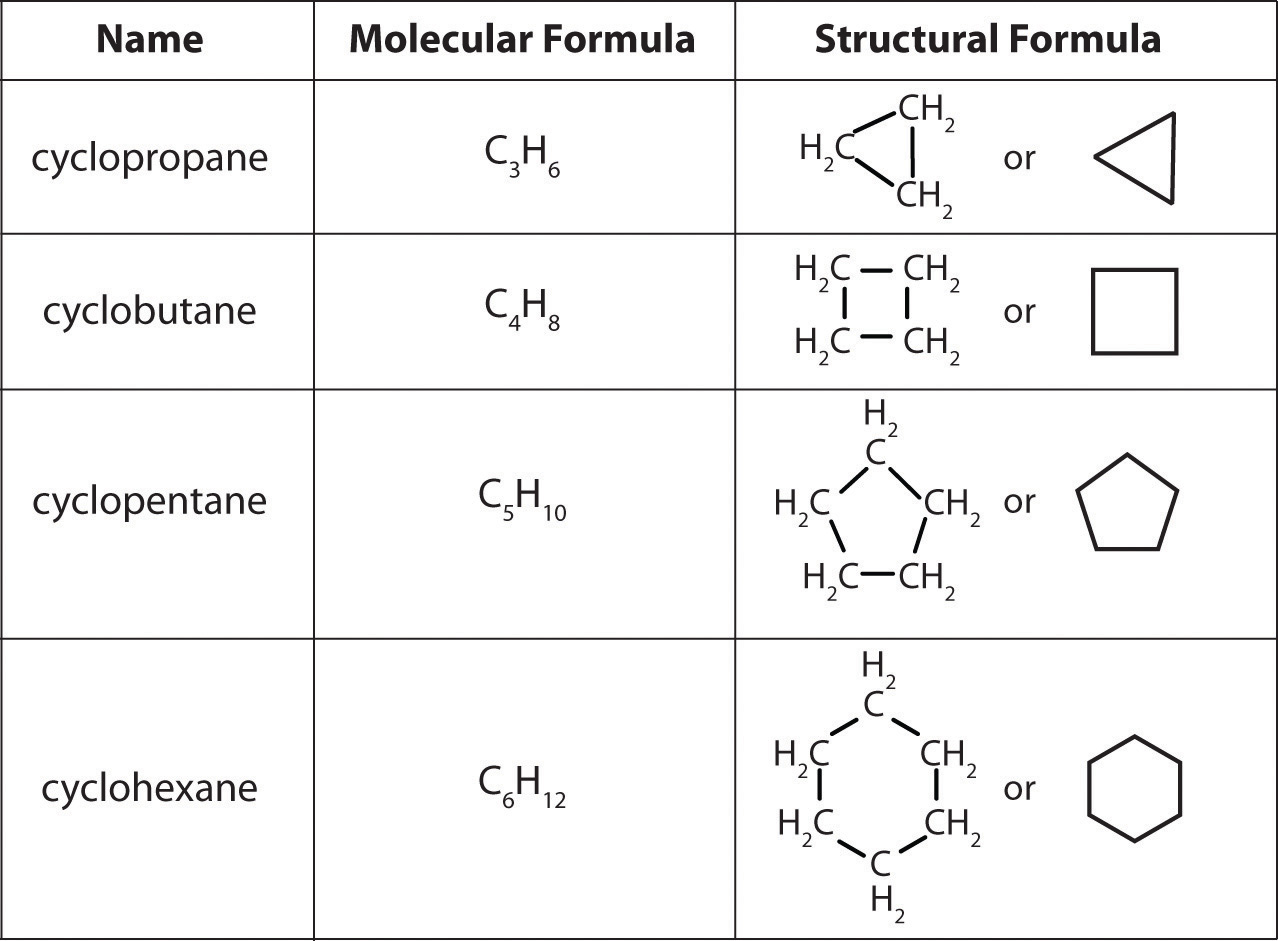
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web draw the lewis structure of the n 2 molecule. Write the correct skeletal structure for the molecule. Different compounds having the same molecular formula are called isomers , and the prevalence of organic isomers reflects the extraordinary versatility of carbon in forming strong.
From Gen Chem to Org Chem, Pt. 7 Lewis Structures Master Organic
Sum the valence electrons from all the atoms. Web drawing lewis structures. 445k views 6 years ago new organic chemistry playlist. What are some examples of lewis structures? * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions.
Drawing organic structures Chemistry for the Health Sciences
Web with a bit of practice, you can easily create lewis structures for almost any molecule. Web this organic chemistry video tutorial shows you how to draw lewis structures, bond line structures and skeletal structures including condensed structural for. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Carbon atoms are depicted.
Lewis Structures in Organic Chemistry Chemistry Steps
Web this organic chemistry video tutorial shows you how to draw lewis structures, bond line structures and skeletal structures including condensed structural for. Web draw out a correct lewis structure for the following compounds. Web drawing lewis structures for simple organic compounds practice | chemistry practice problems | study.com. High school chemistry skills practice. Steps for drawing lewis structures for.
2.9 Some Simple Organic Compounds Chemistry LibreTexts
Carbon atoms are depicted not by a capital c, but by a ‘corner’ between two bonds or a free end of a bond. There are essentially three uses for lewis structures. The methods reviewed above for drawing lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when.
Web It Is Necessary To Draw Structural Formulas For Organic Compounds Because In Most Cases A Molecular Formula Does Not Uniquely Represent A Single Compound.
The example is for the nitrate ion. They’re good at helping you get acquainted with the placement of electrons around atoms, helping to visualize molecular geometry, and finally to remember the location of the lone pairs. In short, these are the steps you need to follow for drawing a lewis structure: Let's consider nitromethane shown below.
Sum The Valence Electrons From All The Atoms.
* hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Web by james ashenhurst. Count the number of carbons and the number of hydrogens. Web here are the steps to draw a lewis structure.
High School Chemistry Skills Practice.
Web common bonding patterns in organic structures. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Different compounds having the same molecular formula are called isomers , and the prevalence of organic isomers reflects the extraordinary versatility of carbon in forming strong bonds to. Web with a bit of practice, you can easily create lewis structures for almost any molecule.
For More Complicated Polyatomic Molecules And Ions, The Lewis Structures Cannot Be Obtained By Simply Combining Lewis Symbols.
This organic chemistry video tutorial explains how to draw lewis structures using a simple method. What are some examples of lewis structures? Molecules can be represented using lewis structures, which show how electrons are arranged around the atoms in a molecule as bonded pairs of electrons (bonds) and lone pairs of electrons. Web this organic chemistry video tutorial shows you how to draw lewis structures, bond line structures and skeletal structures including condensed structural for.