Drawing Molecular Orbital
Drawing Molecular Orbital - Considers bonds as localized between one pair of atoms. Determine the total number of valence electrons in the he 2 2 + ion. This different from nitrogen, where it's the othe. Web molecular orbital theory. As a rule of thumb, a bond order = 1 equates to a single bond, a bond order = 2 equates to a double bond, etc. N = # electrons in bonding orbitals. The molecular orbital (mo) theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. Web chad provides a comprehensive lesson on molecular orbital theory. You're not gonna find these rules anywhere because i made most of them up trying to look at all the different types of molecular orbital's you might have to arrange and figuring out rules that would work for all of them. Web so guys here are seven rules for drawing molecular orbital's and just a heads up.
The disjoint nbmo method involves creating nbmos and connecting them in a disjointed manner, which leaves the. Considers electrons delocalized throughout the entire molecule. As atoms bond to form molecules, a certain number of atomic orbitals combine to form. Molecular orbitals for the h 2 molecule. While the valence bond theory and lewis structures sufficiently explain simple models, the molecular orbital theory provides answers to more complex questions. In the molecular orbital theory, the electrons are delocalized. Web how to draw molecular orbital diagrams for conjugated systems Web sidra ayub (ucd) 7.3: Mo theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process called linear combination of atomic orbitals (lcao). Be sure to obey the pauli principle and hund’s rule while doing so.
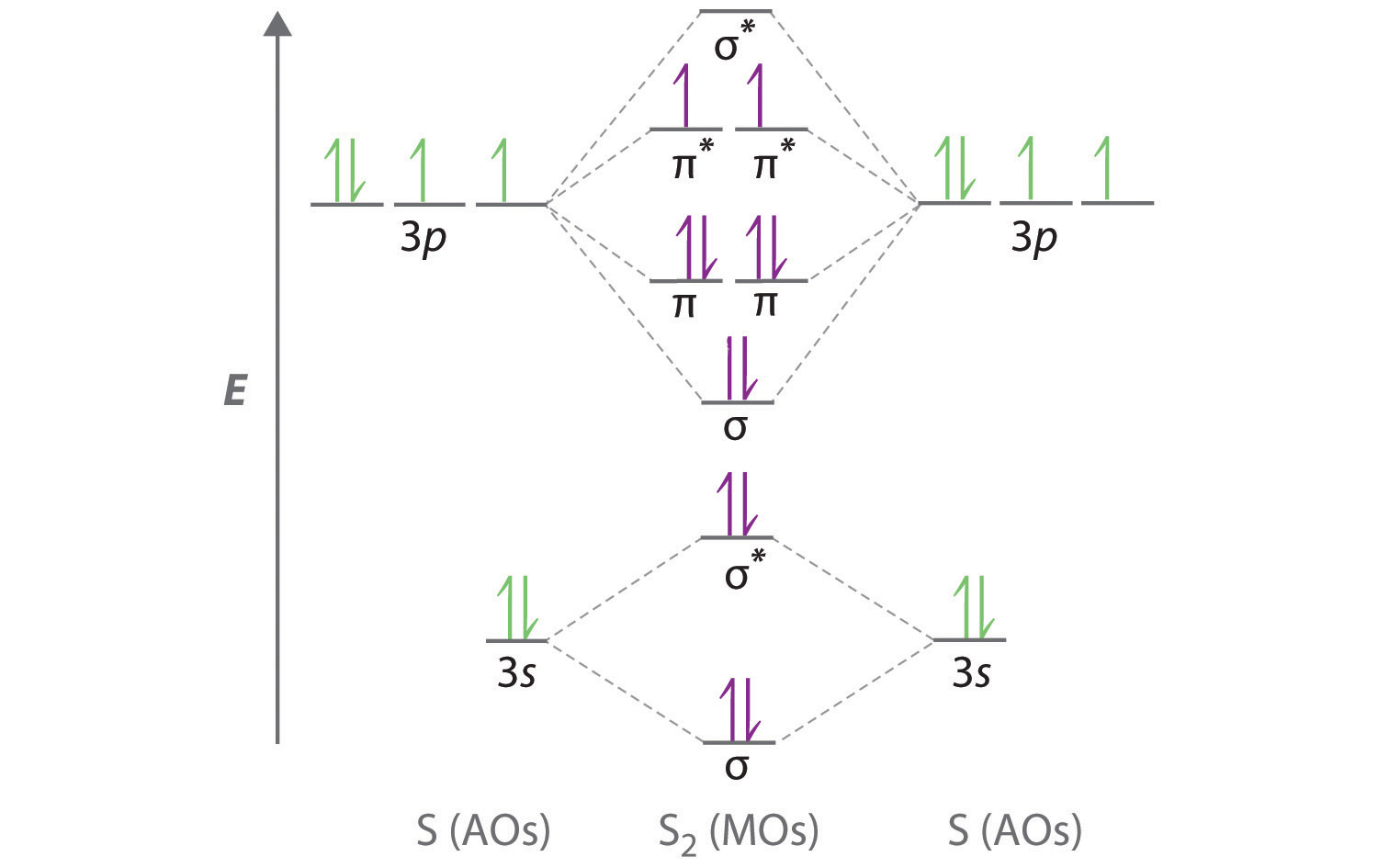
Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding. Determine the total number of valence electrons in the he 2 2 + ion. Web a molecule must have as many molecular orbitals as there are atomic orbitals. The disjoint nbmo method involves creating nbmos and connecting them in a disjointed manner, which leaves the. As atoms bond to form molecules, a certain number of atomic orbitals combine to form. Considers bonds as localized between one pair of atoms. It possible to draw desirable connections among πelectron orbitals.17) a relevant strategy is the design of zero modes, which are nbmos with topological origins. Web molecular orbital diagrams. Each oxygen atom contributes six electrons, so the diagram appears as shown in figure 7.7.15. Bond order gives you an idea of the strength of the bond between two atoms.
9.3 Molecular Orbital Theory Chemistry LibreTexts
Mo theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process called linear combination of atomic orbitals (lcao). Web sign principle is the nonbonding molecular orbital (nbmo). N = # electrons in bonding orbitals. Web we draw a molecular orbital energy diagram similar to that shown in figure.
6.6 3D Representation of Orbitals Chemistry LibreTexts
You're not gonna find these rules anywhere because i made most of them up trying to look at all the different types of molecular orbital's you might have to arrange and figuring out rules that would work for all of them. The applications of the mo theory extend. This different from nitrogen, where it's the othe. The disjoint nbmo method.
10.5 Molecular Orbital Theory Chemistry LibreTexts
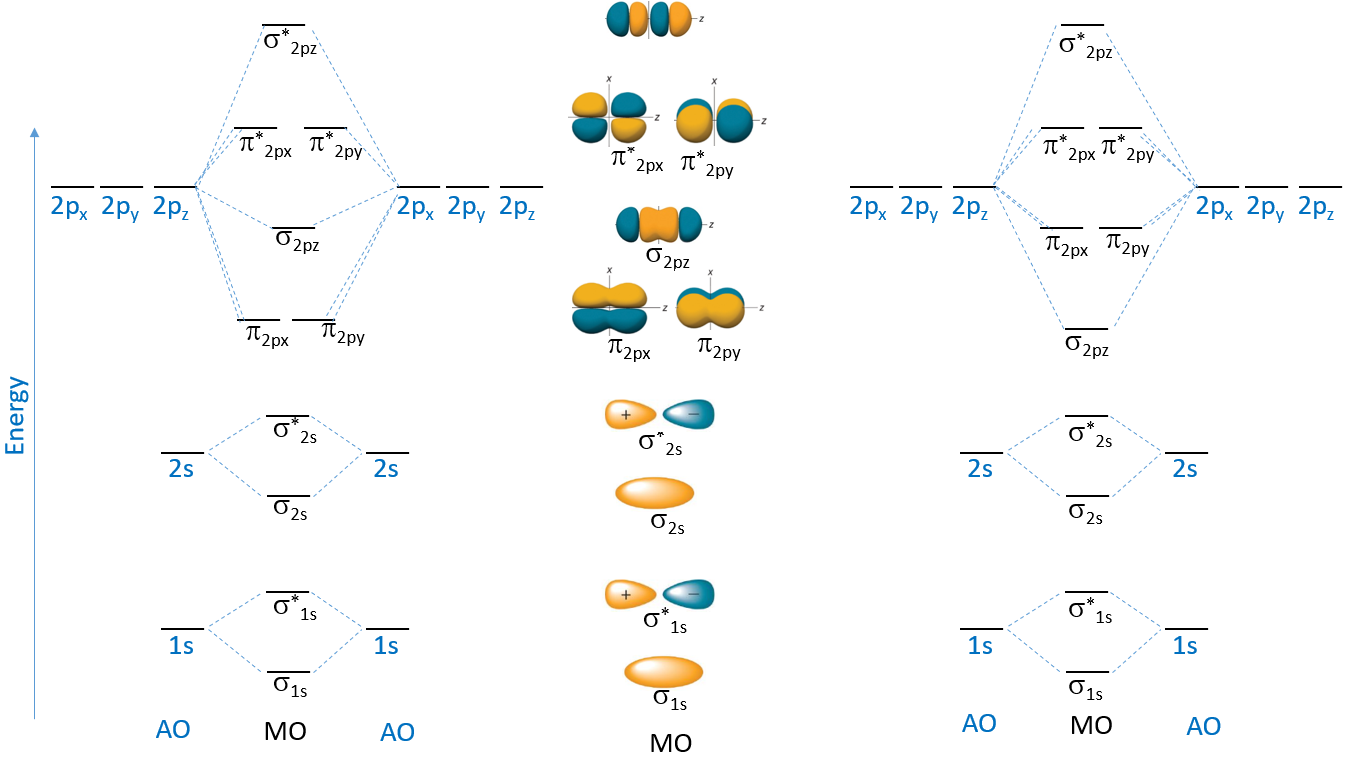
This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2 +. Now some of them are gonna be so. Web sidra ayub (ucd) 7.3: Web we draw a molecular orbital energy diagram similar to that shown in figure 7.7.12. This different from nitrogen,.
Molecular Orbital Theory Chemistry
Molecular orbitals for the h 2 molecule. Web a molecule must have as many molecular orbitals as there are atomic orbitals. In the molecular orbital theory, the electrons are delocalized. Abstract (tl;dr) molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. The disjoint nbmo method involves.
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
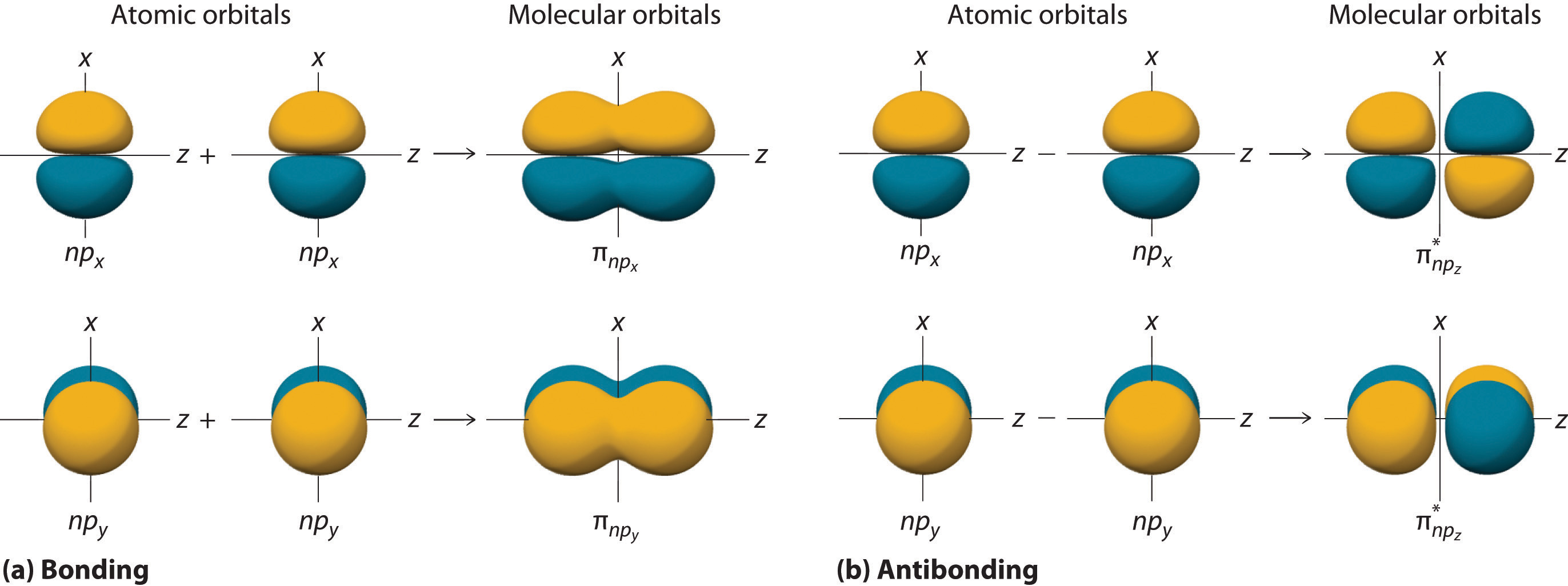
(a) this diagram shows the formation of a bonding σ 1s molecular orbital for h 2 as the sum of the wave functions (ψ) of two h 1. Web in molecular orbital theory, the bonding between atoms is described as a combination of their atomic orbitals. The lesson begins by showing how overlap of atomic orbitals results in both bonding.
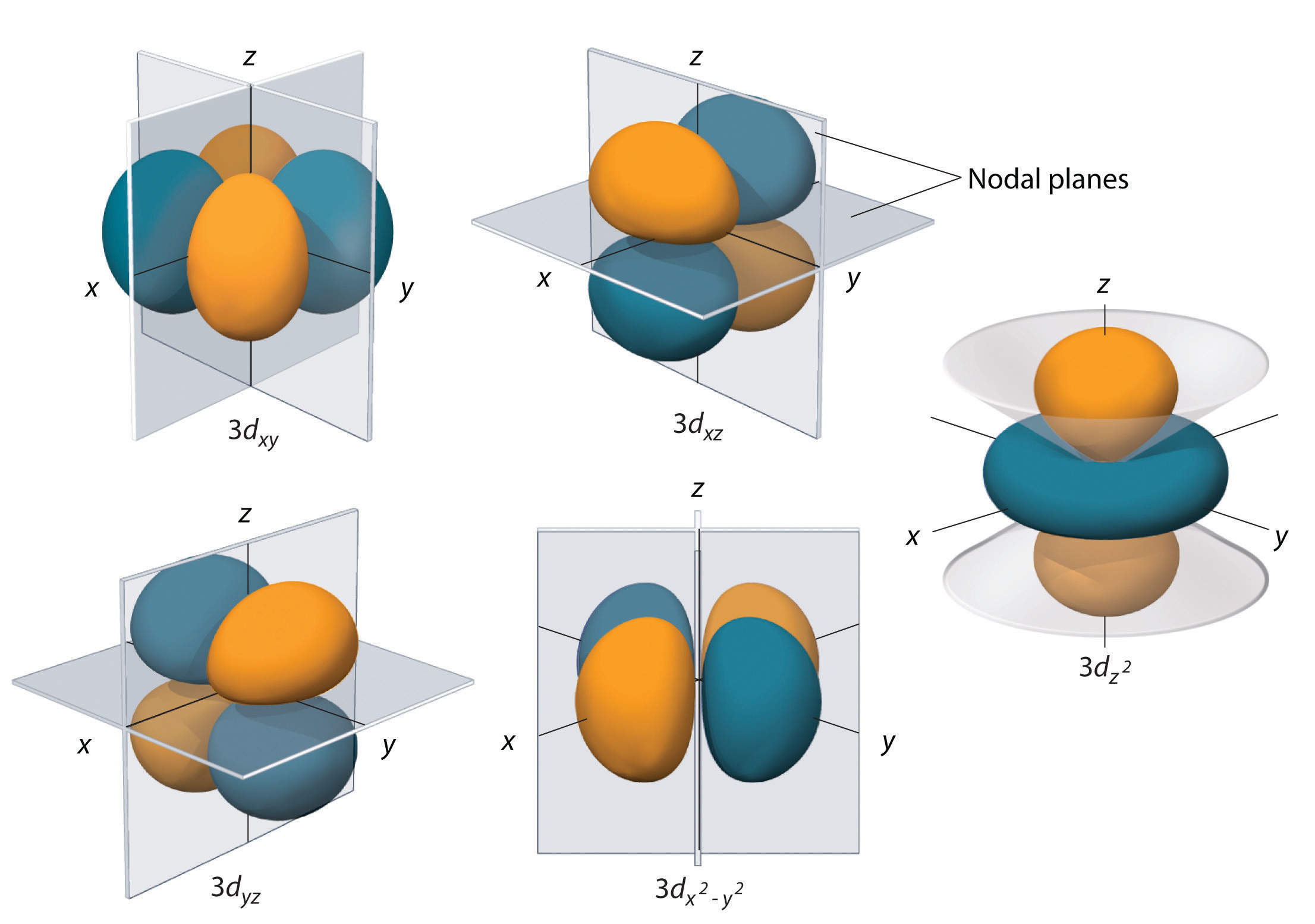
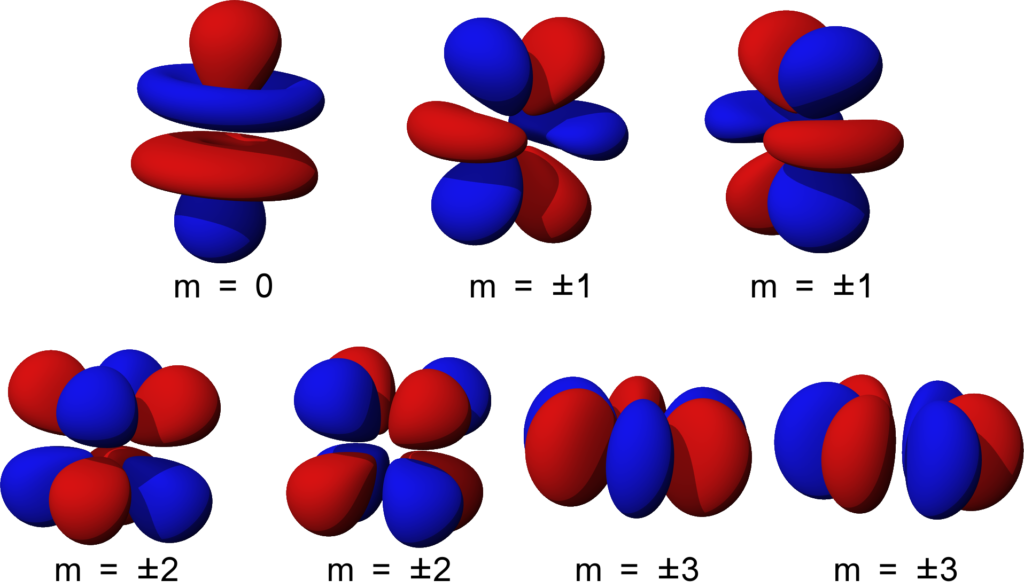
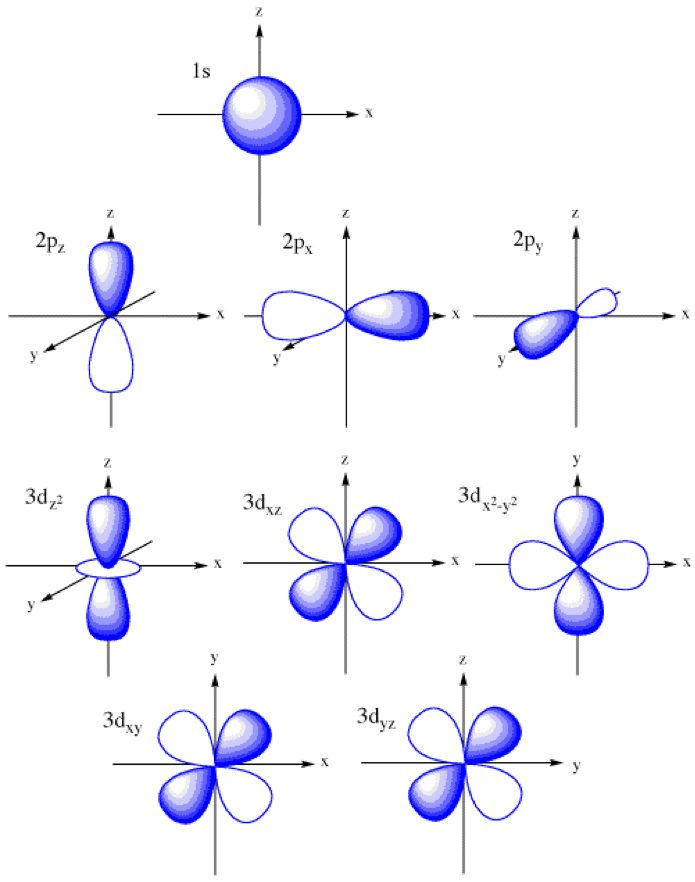
Shapes of Orbitals and their Types Chemistry Skills
Web sidra ayub (ucd) 7.3: Web sign principle is the nonbonding molecular orbital (nbmo). Web molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. The applications of the mo theory extend. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals.
Molecular Orbital Diagram For Cl2
Web how to draw molecular orbital diagrams for conjugated systems In the molecular orbital theory, the electrons are delocalized. Greater overlap = greater change in. Molecular orbitals share many similarities. Construct a qualitative molecular orbital diagram for chlorine, cl 2.
Atomic orbitals explained polizhuge
Web chad provides a comprehensive lesson on molecular orbital theory. (a) this diagram shows the formation of a bonding σ 1s molecular orbital for h 2 as the sum of the wave functions (ψ) of two h 1. Web learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Considers bonds as localized.
energy Molecular orbital diagram for nitrogen monoxide, the nitrosyl
As atoms bond to form molecules, a certain number of atomic orbitals combine to form. Bond order gives you an idea of the strength of the bond between two atoms. Web we draw a molecular orbital energy diagram similar to that shown in figure 7.7.12. Creates bonds from overlap of atomic orbitals ( s, p, d.) and hybrid orbitals (.
Molecular Orbital Diagrams simplified by Megan Lim Medium
As a rule of thumb, a bond order = 1 equates to a single bond, a bond order = 2 equates to a double bond, etc. Considers bonds as localized between one pair of atoms. Web learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. (a) this diagram shows the formation of.
Web Chad Provides A Comprehensive Lesson On Molecular Orbital Theory.
The lesson begins by showing how overlap of atomic orbitals results in both bonding and an. In the molecular orbital theory, the electrons are delocalized. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2 +. Web sign principle is the nonbonding molecular orbital (nbmo).
You're Not Gonna Find These Rules Anywhere Because I Made Most Of Them Up Trying To Look At All The Different Types Of Molecular Orbital's You Might Have To Arrange And Figuring Out Rules That Would Work For All Of Them.
Molecular orbitals for the h 2 molecule. Web a molecule must have as many molecular orbitals as there are atomic orbitals. While the valence bond theory and lewis structures sufficiently explain simple models, the molecular orbital theory provides answers to more complex questions. Based on the amount of orbital overlap, the relative changes in energy differ going from the atomic orbital to the molecular orbital.
Abstract (Tl;Dr) Molecular Orbital Diagrams Are A Fantastic Way Of Visualizing How Molecular Orbitals Form Using What We Already Understand About Sigma And Pi Bonds.
Determine the total number of valence electrons in the he 2 2 + ion. Web molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. Bond order gives you an idea of the strength of the bond between two atoms. Mo theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process called linear combination of atomic orbitals (lcao).
The Applications Of The Mo Theory Extend.
N = # electrons in bonding orbitals. Web so guys here are seven rules for drawing molecular orbital's and just a heads up. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. Web molecular orbital diagrams.