F Orbital Drawing
F Orbital Drawing - This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. They are typically drawn as 3d space around the nucleus and there are different atomic orbital shapes. As you have probably figured by now, the first f orbitals appear in the n=4 shell, and they have three nodal surfaces. For a #p# orbital, draw a figure eight; Each has its own specific energy level and properties. This article will explore the basics of how to draw each type of diagram, and important rules to. Web the impossibility of drawing orbits for electrons. You can't do this for electrons. Web there are four types of orbitals, each with a different shape and represented by the letters s, p, d, and f. It explores s and p orbitals in some detail, including their shapes and energies.
For an #f# orbital, see below. Every unique orbital can only contain up to two electrons. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. 1s, 2s, 2p 3s, 3p,4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. These orbitals are filled with electrons (the amount of electrons depends on which element you are looking at). Web shapes of the 4f orbitals in 3d. Web there are four types of orbitals, each with a different shape and represented by the letters s, p, d, and f. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Web an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. An #s# orbital is a sphere.
Web ask you to learn the f orbital shapes. 1.3k views 5 years ago. There are seven f orbitals and these are the most complicated. Because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated with it, the orbital energy. The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. To do that we need to find the number of electrons for the f atom (there. The square of the orbital wave function represents the probability of finding an electron. Web an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Get a 10 bullets summary of the topic.
F Orbital Shape Definitions, Orbital Chemistry, Atomic Orbitals
A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. Electron shells consist of one or more subshells, and subshells consist of one or more atomic.
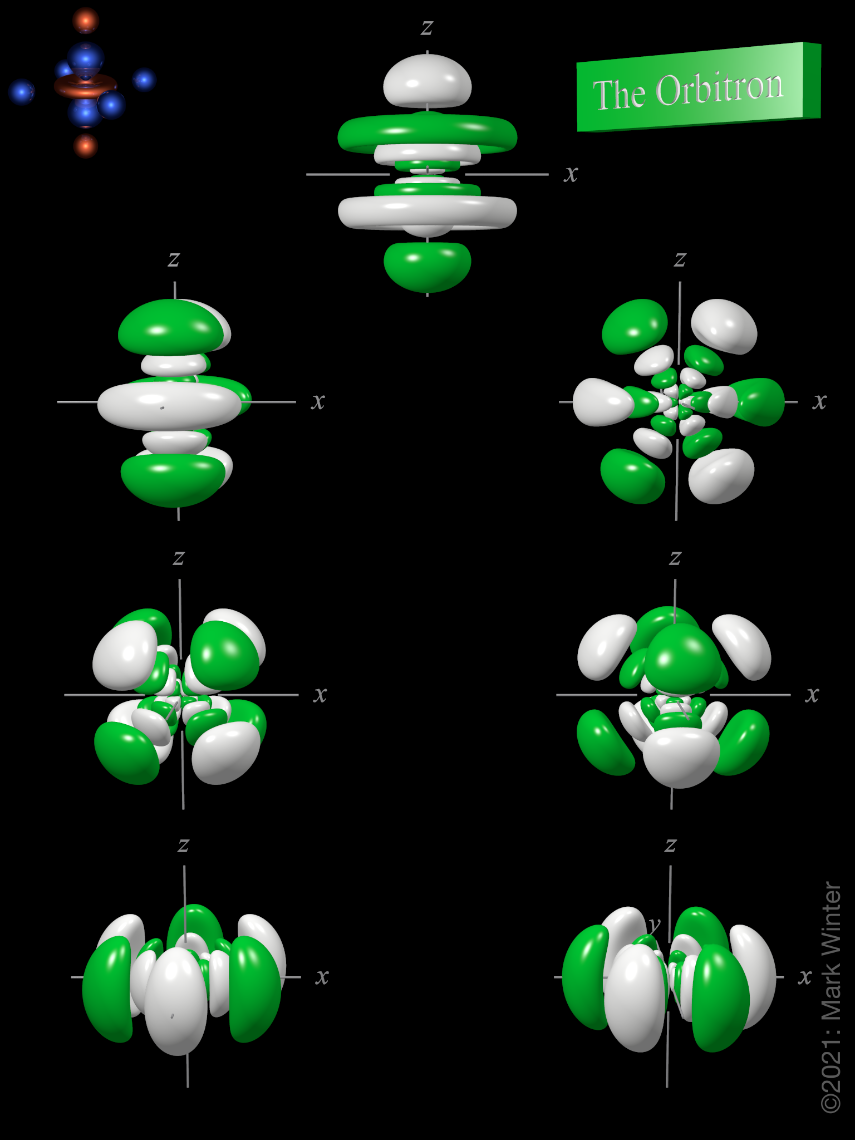
The Orbitron 7f atomic orbitals
Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. An #s# orbital is a sphere. D orbitals are described only in terms of their energy, and f orbitals are only mentioned in passing. This wave function also helps us. Each orbital is denoted by a number and a letter.
Shapes of Orbitals and their Types Chemistry Skills
Because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated with it, the orbital energy. You can't do this for electrons. Web what are s,p,d,f orbitals? There are multiple orbitals within an atom. One reason for this is that the f orbitals.
F Orbital Shape Definitions, Orbital Chemistry, Atomic Orbitals
There are multiple orbitals within an atom. Orbitals are the regions of space in which electrons are most likely to be found. For a #p# orbital, draw a figure eight; However, the orbital shapes can be useful in interpreting spectra and in understanding the structure of some complexes that involve the rare earth elements. Electron shells consist of one or.
What are the different kinds of f orbitals? Socratic
This article will explore the basics of how to draw each type of diagram, and important rules to. It explores s and p orbitals in some detail, including their shapes and energies. Web for an #s# orbital, draw a circle; Because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set.
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
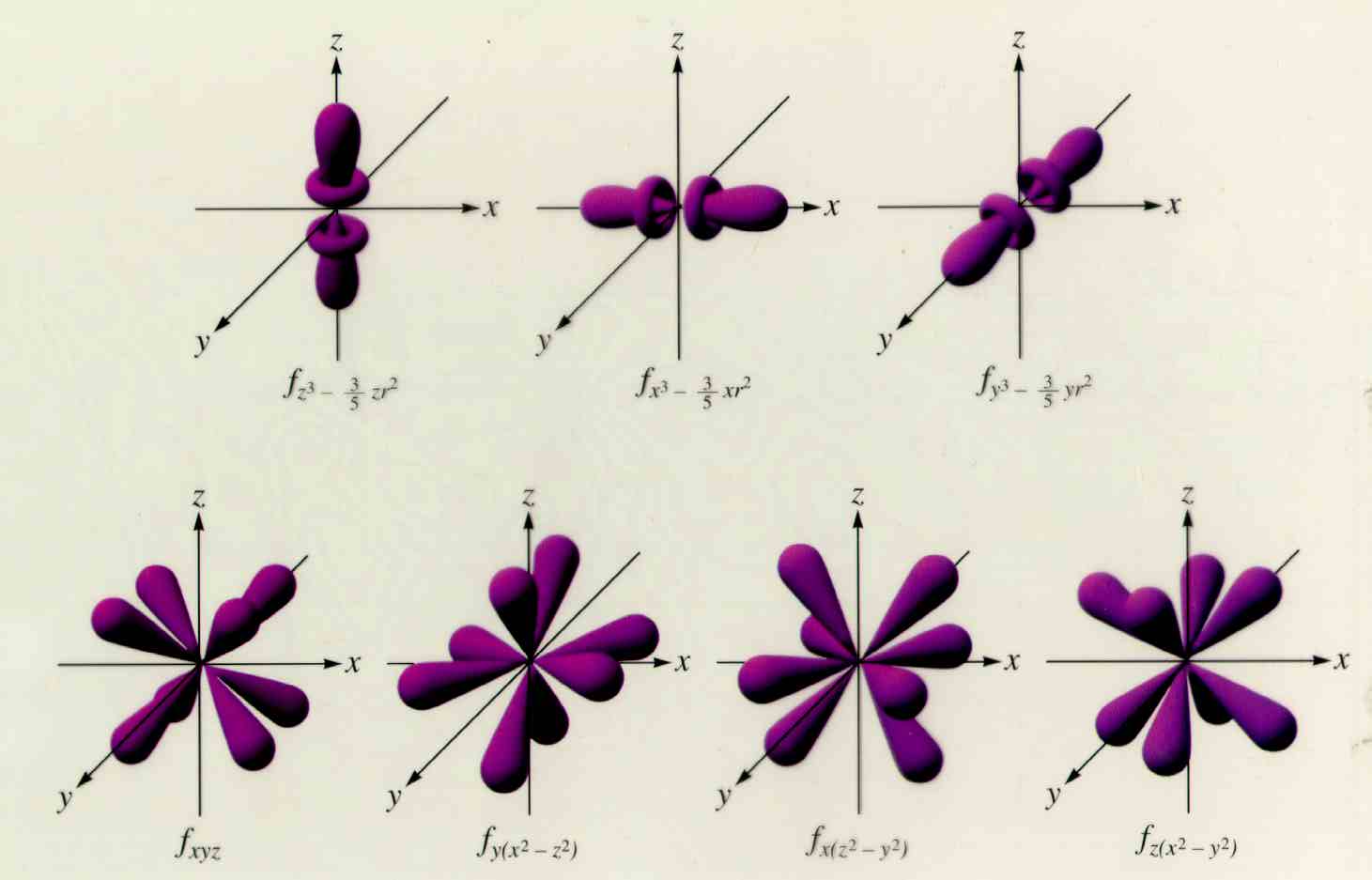
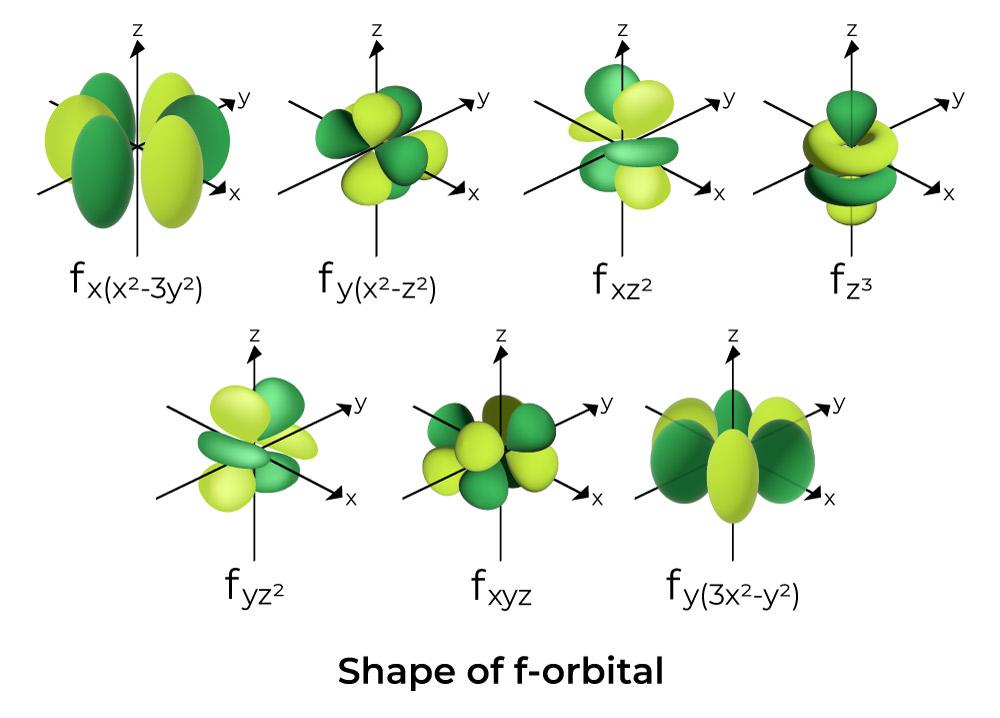
Web ask you to learn the f orbital shapes. Every unique orbital can only contain up to two electrons. Web orbitals with \(\ell = 3\) are f orbitals, which are still more complex. Web an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins.
What is the shape of forbital??? + Example
A p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. Web this page discusses atomic orbitals at an introductory level. Get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. Click the images to see the various 4f orbitals. Because its.
F Orbital Shape Definitions, Orbital Chemistry, Atomic Orbitals
Web to write the orbital diagram for the fluorine atom (f) first we need to write the electron configuration for just f. This article will explore the basics of how to draw each type of diagram, and important rules to. These seven orbitals have the following ml values: The number denotes the energy level of the electron in the orbital..
F Orbital Shape Definitions, Orbital Chemistry, Atomic Orbitals
There are multiple orbitals within an atom. Web a p orbital which extends along the x axis is labeled a p x orbital. Each has its own specific energy level and properties. Because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated.
Shapes of Atomic Orbitals Shape of s, p, d, f Orbitals, FAQs, Examples
This wave function also helps us. To do that we need to find the number of electrons for the f atom (there. One reason for this is that the f orbitals are very little used in any chemical bonds. These seven orbitals have the following ml values: Web there are four types of orbitals, each with a different shape and.
Web Quantum Numbers Describing Electronic Orbitals.
Web there are four types of orbitals, each with a different shape and represented by the letters s, p, d, and f. The square of the orbital wave function represents the probability of finding an electron. However, the orbital shapes can be useful in interpreting spectra and in understanding the structure of some complexes that involve the rare earth elements. Atomic orbitals describe the most likely location that electrons will be found around the nucleus of an atom.
Web To Write The Orbital Diagram For The Fluorine Atom (F) First We Need To Write The Electron Configuration For Just F.
Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Each orbital is denoted by a number and a letter. Web this page discusses atomic orbitals at an introductory level. Orbitals are the regions of space in which electrons are most likely to be found.
They Are Typically Drawn As 3D Space Around The Nucleus And There Are Different Atomic Orbital Shapes.
This article will explore the basics of how to draw each type of diagram, and important rules to. Each has its own specific energy level and properties. Because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated with it, the orbital energy. Because each orbital is different, they are assigned specific quantum numbers:
As You Have Probably Figured By Now, The First F Orbitals Appear In The N=4 Shell, And They Have Three Nodal Surfaces.
D orbitals are described only in terms of their energy, and f orbitals are only mentioned in passing. There are multiple orbitals within an atom. Click the images to see the various 4f orbitals. These orbitals are filled with electrons (the amount of electrons depends on which element you are looking at).