Fda Catalyst Calendar
Fda Catalyst Calendar - Food and drug administration (fda) accepted the supplemental new drug application (snda) for lumryz for treatment of cataplexy or eds in the pediatric. Comprehensive suite of tools for trading and investing in. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions. Web the calendar lists down all key catalysts that can materially impact stocks, including: Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Web with the catalyst calendar, people can filter and search by disease, molecular target, as well as date, company, product name and event type. Web trial tracker is an intuitive tool that helps you investigate companies facing upcoming clinical trial catalysts. With daily updates, you'll always have. Web catacal is a catalyst calendar that reveals impactful stock market catalyst events. Web calendar of fda public advisory committee meetings.
Web our historical fda catalyst calendar lists all drug catalysts that have already occurred, dating back to 2009. Note that we do not include phase 1 catalysts in this calendar. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Web better intelligence tools for regulatory, financial, and clinical trial catalysts Event types range from product. Web with the catalyst calendar, people can filter and search by disease, molecular target, as well as date, company, product name and event type. Web trial tracker is an intuitive tool that helps you investigate companies facing upcoming clinical trial catalysts. Web comprehensive suite of tools for trading and investing in biotech stocks. Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with rttnews fda calendar & upcoming approvals. Pdufa target dates are dates by which the fda aims to deliver their.
Web with the catalyst calendar, people can filter and search by disease, molecular target, as well as date, company, product name and event type. Web comprehensive suite of tools for trading and investing in biotech stocks. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Pdufa target dates are dates by which the fda aims to deliver their. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Food and drug administration (fda) accepted the supplemental new drug application (snda) for lumryz for treatment of cataplexy or eds in the pediatric. Web this article will detail the catalysts which should become part of your clinical and fda calendar for each biotech stock you're trading or looking to invest in. Web calendar of fda public advisory committee meetings. Web comprehensive suite of tools for trading and investing in biotech stocks. Web better intelligence tools for regulatory, financial, and clinical trial catalysts
FDA Calendar FDA Tracker
Fda calendar, pdufa date calendar, biotech company screener and database and much more. Fda calendar, pdufa date calendar, biotech company screener and database and much more Web comprehensive suite of tools for trading and investing in biotech stocks. Web comprehensive suite of tools for trading and investing in biotech stocks. Comprehensive suite of tools for trading and investing in.
FDA/PDUFA Catalyst Calendar (*Updated) endMarch/April 2024 and Highest
Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). Web comprehensive suite of tools for trading and investing in biotech stocks. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions. Pdufa dates, or in other words fda decision dates. Web better intelligence tools.
Biotech and Pharma FDA/Catalyst Calendar for mainly the last 2
Web comprehensive suite of tools for trading and investing in biotech stocks. Pdufa dates, or in other words fda decision dates. Web catacal is a catalyst calendar that reveals impactful stock market catalyst events. Web this article will detail the catalysts which should become part of your clinical and fda calendar for each biotech stock you're trading or looking to.
FDA/PDUFA Catalyst Calendar (*Updated) midMarch 2024 and Highest
Fda calendar, pdufa date calendar, biotech company screener and database and much more Web comprehensive suite of tools for trading and investing in biotech stocks. Web trial tracker is an intuitive tool that helps you investigate companies facing upcoming clinical trial catalysts. Pdufa target dates are dates by which the fda aims to deliver their. Web the fda pdufa report.
Biotech and Pharma FDA/Catalyst Calendar for mainly the last 2
Web trial tracker is an intuitive tool that helps you investigate companies facing upcoming clinical trial catalysts. Fda calendar, pdufa date calendar, biotech company screener and database and much more Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Note that we.
FDA/PDUFA Catalyst Calendar (*Updated) endMarch/April 2024 and Highest
Web with the catalyst calendar, people can filter and search by disease, molecular target, as well as date, company, product name and event type. Web better intelligence tools for regulatory, financial, and clinical trial catalysts Web therapy for hereditary angioedema (hae). Pdufa dates, or in other words fda decision dates. Web our enhanced fda calendar integrates pdufa dates, clinical trial.
FDA/PDUFA Catalyst Calendar (*Updated) midMarch 2024 and Highest
Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). Web comprehensive suite of tools for trading and investing in biotech stocks. Web with the catalyst calendar, people can filter and search by disease, molecular target, as well as date, company, product name and event type. Web get daily updates on.
Biotech/Pharma SqueezeFinder with FDA Catalyst Calendar (*Updated) r
Fda calendar, pdufa date calendar, biotech company screener and database and much more Web comprehensive suite of tools for trading and investing in biotech stocks. Web better intelligence tools for regulatory, financial, and clinical trial catalysts Event types range from product. Fda calendar, pdufa date calendar, biotech company screener and database and much more.
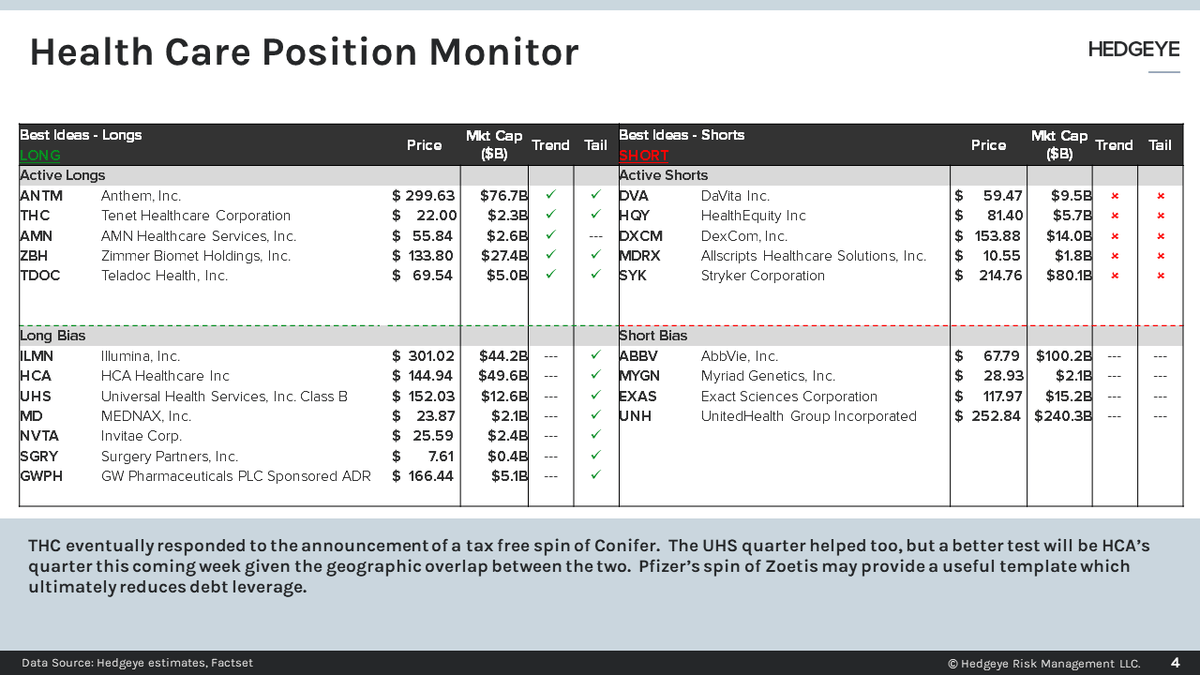
Hedgeye Position Monitor Live Q&A THC Spinco DVA Catalyst
Fda calendar, pdufa date calendar, biotech company screener and database and much more Companies can be screened by market cap and stock symbol;. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Pdufa dates, or in other words fda decision dates..
Biotech Weekly FDA Catalyst Watchlist Jan 2nd 2022
Fda calendar, pdufa date calendar, biotech company screener and database and much more. Web therapy for hereditary angioedema (hae). Web comprehensive suite of tools for trading and investing in biotech stocks. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web our fda calendar is.
Web Trial Tracker Is An Intuitive Tool That Helps You Investigate Companies Facing Upcoming Clinical Trial Catalysts.
Web better intelligence tools for regulatory, financial, and clinical trial catalysts Note that we do not include phase 1 catalysts in this calendar. Comprehensive suite of tools for trading and investing in. Fda calendar, pdufa date calendar, biotech company screener and database and much more.
With Daily Updates, You'll Always Have.
Pdufa dates, or in other words fda decision dates. Companies can be screened by market cap and stock symbol;. Event types range from product. Web comprehensive suite of tools for trading and investing in biotech stocks.
Web Our Fda Calendar Is Designed To Provide You With Future Catalysts Across Biotech & Pharma Companies, Updated On A Daily Basis For All Companies We Cover.
Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with rttnews fda calendar & upcoming approvals. Web calendar of fda public advisory committee meetings.
Web With The Catalyst Calendar, People Can Filter And Search By Disease, Molecular Target, As Well As Date, Company, Product Name And Event Type.
Web the calendar lists down all key catalysts that can materially impact stocks, including: Pdufa target dates are dates by which the fda aims to deliver their. Food and drug administration (fda) accepted the supplemental new drug application (snda) for lumryz for treatment of cataplexy or eds in the pediatric. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions.