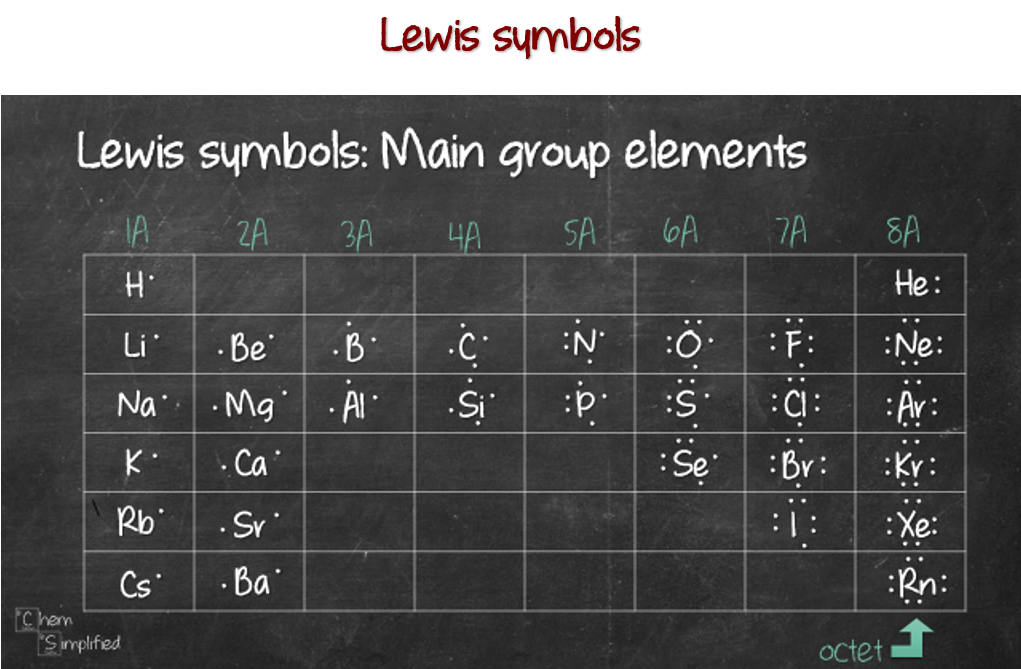
How Many Bonds Can Bromine Form
How Many Bonds Can Bromine Form - Group 5a (15) elements such as nitrogen have five valence electrons in. 1 bond how many bonds can sulfur form with neighboring atoms in a compound? Web how many bonds can bromine form with neighboring atoms in a compound? Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet Two single bonds and a double bond. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next When bromine atoms form covalent bonds with other atoms,. A single bond and two double bonds. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Modeling lonic and covalent bonds part 1:
1 bond how many bonds can sulfur form with neighboring atoms in a compound? Web how many bonds will bromine most likely form?4 , 2, 1, or 7. Web bromine will normally form one covalent bond. View the full answer transcribed image text: The valence electrons in al is three. Let's illustrate how a covalent bond forms between. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet Web expert answer 100% (6 ratings) carbon atom has four valence electr. Web how many bonds can bromine make? Web bromine is capable of forming one bond when it is in its elemental form.
Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web bromine is capable of forming one bond when it is in its elemental form. Web how many bonds will bromine most likely form?4 , 2, 1, or 7. When bromine atoms form covalent bonds with other atoms,. Two single bonds and a double bond. Web bromine compounds are compounds containing the element bromine (br). Let's illustrate how a covalent bond forms between. View the full answer transcribed image text:
HONC 1234 ChemSimplified
For example, it can form two bonds with. 1 pm = 1 × 10 −12 m). Web bromine is capable of forming one bond when it is in its elemental form. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Web how many bonds can bromine form with neighboring atoms.
How Can We Find A Electron Configuration For Bromine (Br)
Web bromine compounds are compounds containing the element bromine (br). Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Let's illustrate how a covalent bond forms between. Web bromine will typically form one bond, as it is a halogen. Web how many bonds can bromine make?
According to the following reaction, how... Chemistry
Web how many bonds will bromine most likely form?4 , 2, 1, or 7. Which of the following situations meet the bonding requirement for carbon atoms. When bromine atoms form covalent bonds with other atoms,. Modeling lonic and covalent bonds part 1: Web expert answer 100% (6 ratings) carbon atom has four valence electr.
How Many Bonds Can Nitrogen Form Jacks Of Science
Web expert answer 100% (6 ratings) carbon atom has four valence electr. 1 bond how many bonds can sulfur form with neighboring atoms in a compound? The maximum number of bonds. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Web bromine is capable of forming one bond when it.
Solved how many bonds can/should be drawn to the following
Web bromine will typically form one bond, as it is a halogen. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; Web expert answer 100% (6 ratings) carbon atom has four valence electr. Web bromine exists as a diatomic molecule with the chemical.
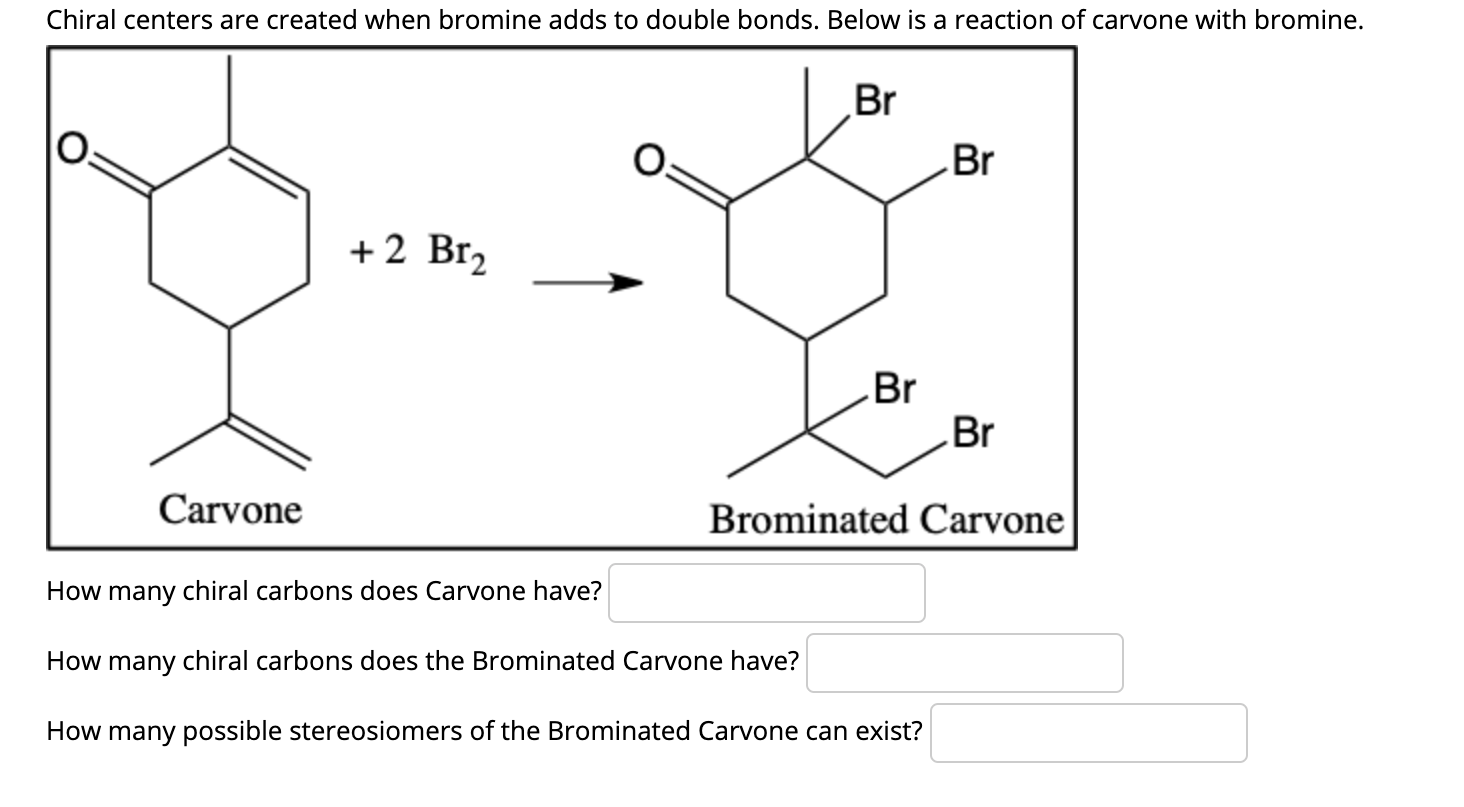
Solved Chiral centers are created when bromine adds to
When bromine atoms form covalent bonds with other atoms,. Two single bonds and a double bond. Web bromine will normally form one covalent bond. 6 which elements tend to form covalent bonds? Modeling lonic and covalent bonds part 1:
How to Predict number of bonds each element forms ChemSimplified
Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web bromine will typically form one bond, as it is a halogen. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet Web the bond in a hydrogen molecule,.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. For example, it can form two bonds with. Web expert answer 100% (6 ratings) carbon atom has four valence electr. 1 bond how many bonds can sulfur form with neighboring atoms in a compound? Web the bond in a hydrogen molecule,.
Physical and Chemical Properties Bromine
Let's illustrate how a covalent bond forms between. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. The valence electrons in al is three. A single bond and two double bonds. 1 bond how many bonds can sulfur form with neighboring atoms in a compound?
How Many Bonds Can Nitrogen Form Jacks Of Science
Web how many bonds can bromine form with neighboring atoms in a compound? Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. View the full answer transcribed image text: Two single bonds and a double bond. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have.
Bromine, Which Belongs To Group 17 And Period Four Of The Periodic Table, Has Seven Outer Shell Or Valence.
6 which elements tend to form covalent bonds? Web how many bonds will bromine most likely form?4 , 2, 1, or 7. Web how many bonds can bromine make? Web expert answer 100% (6 ratings) carbon atom has four valence electr.
Web Bromine Is Capable Of Forming One Bond When It Is In Its Elemental Form.
Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. However, it can also form multiple bonds with other elements. Web bromine will normally form one covalent bond.
Web Let's Illustrate How A Covalent Bond Forms Between Iodine And Bromine, With The Understanding That Each Atom Only Needs One More Electron To Complete An Octet
Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Two single bonds and a double bond. For example, it can form two bonds with. View the full answer transcribed image text:
Web If Either Iodine Or Bromine Were To Given Up Valence Electrons To Form A Cation, They Would Have To Give Up All Seven Valence Electrons To Reveal The Next
1 pm = 1 × 10 −12 m). The maximum number of bonds. Oxygen will normally have tow bonds, although it may have three in certain molecules (ozone is an example). A single bond and two double bonds.