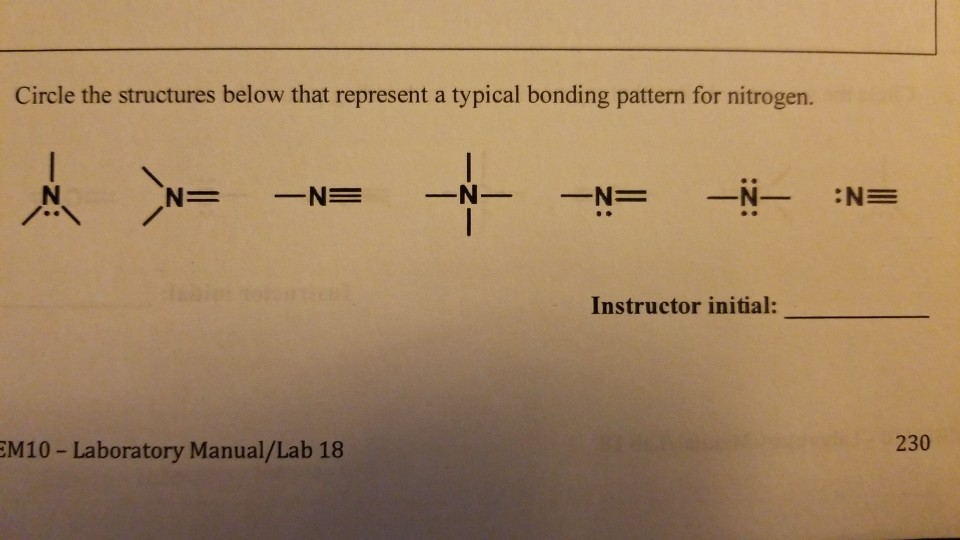
How Many Covalent Bonds Does Nitrogen Form
How Many Covalent Bonds Does Nitrogen Form - This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5. Nitrogen (group 15 or 5a) 3: Web nitrogen can form 3 covalent bonds. Typically, the atoms of group 4a form 4 covalent bonds; Web therefore, nitrogen can form 3 single covalent bonds. Nitrogen has 5 valence electrons. How many covalent bonds will a nitrogen atom usually form? Nitrogen is in group 5 of the periodic table. Will form either one, two, three or four covalent bonds with other atoms. And group 7a form one bond.
Since the small size and high electronegativity allows nitrogen atoms to form multiple bonds,. A nitrogen atom has 5 electrons in its outer shell. Nitrogen has 5 valence electrons. Whether a molecule is polar or nonpolar depends both on bond type and molecular shape. Group 5a form 3 bonds; The formal charge on the bromine atom in bro3. Group 6a form 2 bonds; How many covalent bonds will a nitrogen atom usually form? There is a quick way to work out how many covalent bonds an element will. Nitrogen typically forms 3 covalent bonds, including in n 2.
There is a quick way to work out how many covalent bonds an element will. How many covalent bonds will a nitrogen atom usually form? Web how many covalent bonds does nitrogen form in electrically neutral compounds? Group 6a form 2 bonds; The most common gas in the atmosphere, nitrogen, is made of two nitrogen atoms bonded. Group 5a form 3 bonds; Web nitrogen atoms have five valence electrons, three of which are unpaired originally. Web atoms of different elements. Will form either one, two, three or four covalent bonds with other atoms. Whether a molecule is polar or nonpolar depends both on bond type and molecular shape.
What Happens When Two Nitrogen Atoms Share Electrons MarisolkruwLee
The most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share. Web chemistry questions and answers. The formal charge on the bromine atom in bro3. How many covalent bonds does carbon form in neutral compounds? Web 2h ( g) h 2 ( g) δ h = −436 kj pure vs.
What is Nitrogen? Definition, Formula, Cycle, Fixation
There is a quick way to work out how many covalent bonds an element will. Web atoms of different elements. Nitrogen (group 15 or 5a) 3: Web nitrogen atoms have five valence electrons, three of which are unpaired originally. Web nitrogen is a versatile element and can form a variety of bonds.
__TOP__ How Many Covalent Bonds Can Chlorine Form
This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5. Typically, the atoms of group 4a form 4 covalent bonds; Web nitrogen atoms have five valence electrons, three of which are unpaired originally. Web therefore, nitrogen can form 3 single covalent bonds. Nitrogen is in group 5 of the periodic table.
Covalent Bond Biology Dictionary
Group 5a form 3 bonds; How many covalent bonds does carbon form in neutral compounds? Group 6a form 2 bonds; The most common gas in the atmosphere, nitrogen, is made of two nitrogen atoms bonded. Since the small size and high electronegativity allows nitrogen atoms to form multiple bonds,.
Covalent Bonds Biology for NonMajors I
Web how many covalent bonds does nitrogen form in electrically neutral compounds? How many covalent bonds does carbon form in neutral compounds? Group 6a form 2 bonds; Since the small size and high electronegativity allows nitrogen atoms to form multiple bonds,. The most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share.
Solved 11. Answer each of the following questions (a) Draw
Whether a molecule is polar or nonpolar depends both on bond type and molecular shape. A nitrogen atom has 5 electrons in its outer shell. How many covalent bonds will a nitrogen atom usually form? Web atoms of different elements. There is a quick way to work out how many covalent bonds an element will.
How does a covalent bond form ? Show the formation of nitrogen molecule
Web therefore, nitrogen can form 3 single covalent bonds. Group 5a form 3 bonds; Web chemistry questions and answers. Nitrogen (group 15 or 5a) 3: Web how many covalent bonds does nitrogen form in electrically neutral compounds?
Solved Com How many bonds does nitrogen typically form?
How many covalent bonds does carbon form in neutral compounds? Typically, the atoms of group 4a form 4 covalent bonds; Both water and carbon dioxide have polar covalent bonds, but Whether a molecule is polar or nonpolar depends both on bond type and molecular shape. Web nitrogen is a versatile element and can form a variety of bonds.
How Many Bonds Does Nitrogen Form tour bous
Web how many covalent bonds does nitrogen form in electrically neutral compounds? Don’t get confused as in this question single nitrogen atom (n) is asked not for dinitrogen n 2. Group 5a form 3 bonds; Both water and carbon dioxide have polar covalent bonds, but The formal charge on the bromine atom in bro3.
Solved How many bonds does nitrogen typically form? Does
Whether a molecule is polar or nonpolar depends both on bond type and molecular shape. Nitrogen (group 15 or 5a) 3: Web nitrogen can form 3 covalent bonds. Nitrogen typically forms 3 covalent bonds, including in n 2. Web how many covalent bonds are formed?
The Formal Charge On The Bromine Atom In Bro3.
Nitrogen is in group 5 of the periodic table. Group 6a form 2 bonds; The most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share. There is a quick way to work out how many covalent bonds an element will.
Web Nitrogen Is A Versatile Element And Can Form A Variety Of Bonds.
If nitrogen is to remain neutral complete the following equation. Will form either one, two, three or four covalent bonds with other atoms. Web nitrogen atoms have five valence electrons, three of which are unpaired originally. Polar covalent bonds if the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the.
Typically, The Atoms Of Group 4A Form 4 Covalent Bonds;
Group 6a form 2 bonds; Nitrogen typically forms 3 covalent bonds, including in n 2. A nitrogen atom has 5 electrons in its outer shell. Web in order to form a covalent bond, each element has to share one unpaired electron.
Nitrogen Has 5 Valence Electrons.
Don’t get confused as in this question single nitrogen atom (n) is asked not for dinitrogen n 2. Both water and carbon dioxide have polar covalent bonds, but Group 5a form 3 bonds; For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom.