How To Draw A Mo Diagram
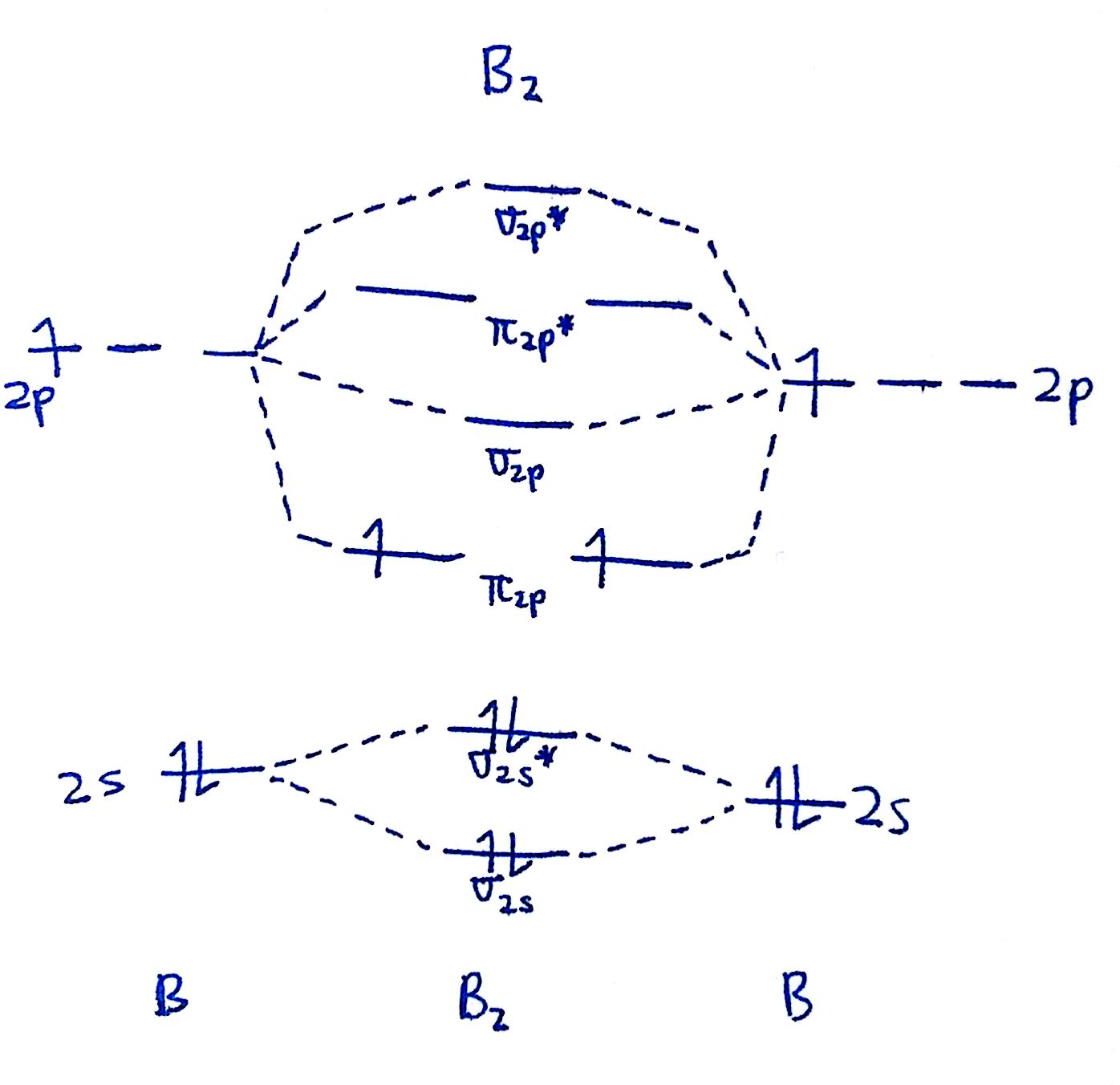
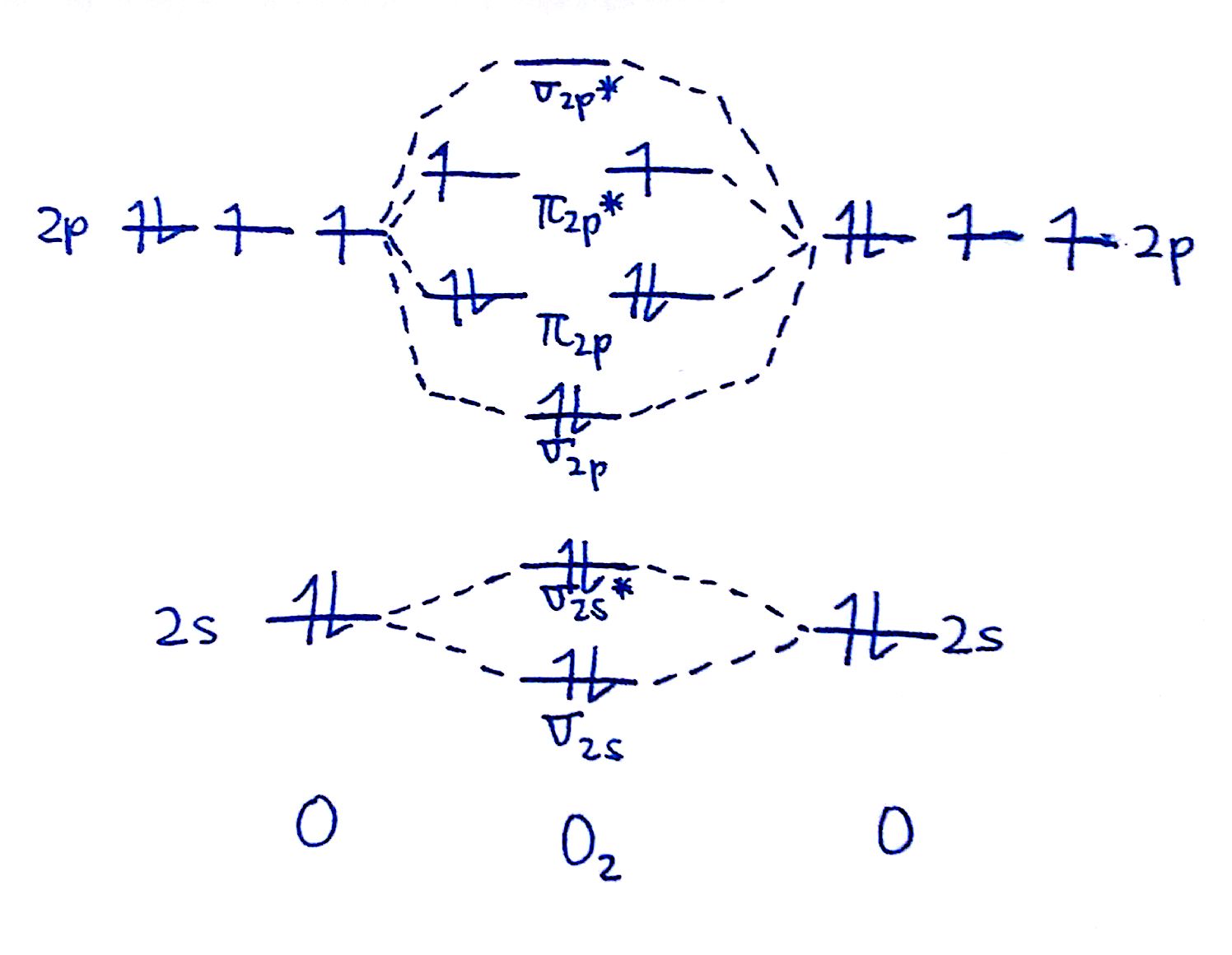
How To Draw A Mo Diagram - Greater overlap = greater change in. Draw the mo diagram for `o_2^+` this is a bit of a curveball, but a perfectly valid problem. The striker was controversially sent off, following a lengthy var review, in. Try the following mo's on your own, and then check with the answers provided. This approach is used only when the group orbitals are not obvious by inspection. Web when two oxygen atoms overlap, the sigma(2p) molecular orbital is lower in energy than the pi(2p) orbitals. That is, it's `o_2` with `1` missing electron. Draw two lines to create three columns. Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. The first major step is understanding the difference between two major theories:
Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Define bond order, and state its significance.; For this example, we will be using chlorine. We need to know what orbitals we are using. Valence bond theory and molecular. Web for chem videos, quizzes and more download chemistry x for free on the app store! The first major step is understanding the difference between two major theories: Draw the mo for o 2: Try the following mo's on your own, and then check with the answers provided.
Make sure you thoroughly understand the following essential ideas. As a rule of thumb, a bond order = 1 equates to a single bond, a bond order = 2 equates to a double bond, etc. We are only going to consider valence orbitals. Greater overlap = greater change in. We will predict their bond order and see how the energies of the different orbitals change. They are usually used to explore domain concepts, understand software requirements and. The first major step is understanding the difference between two major theories: Web drawing molecular orbital diagrams is one of the trickier concepts in chemistry. Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. Draw the mo diagram for n2:
How to Draw Molecular Orbital Diagrams (MO DIAGRAMS) Explanation YouTube
The mo diagram will be the same as the mo diagram of `o_2`, except with `1` less electron. Determine point group of molecule (if linear, use d. H has a 1s orbital. From this diagram, calculate the bond order for o 2. Greater overlap = greater change in.
MO Diagrams
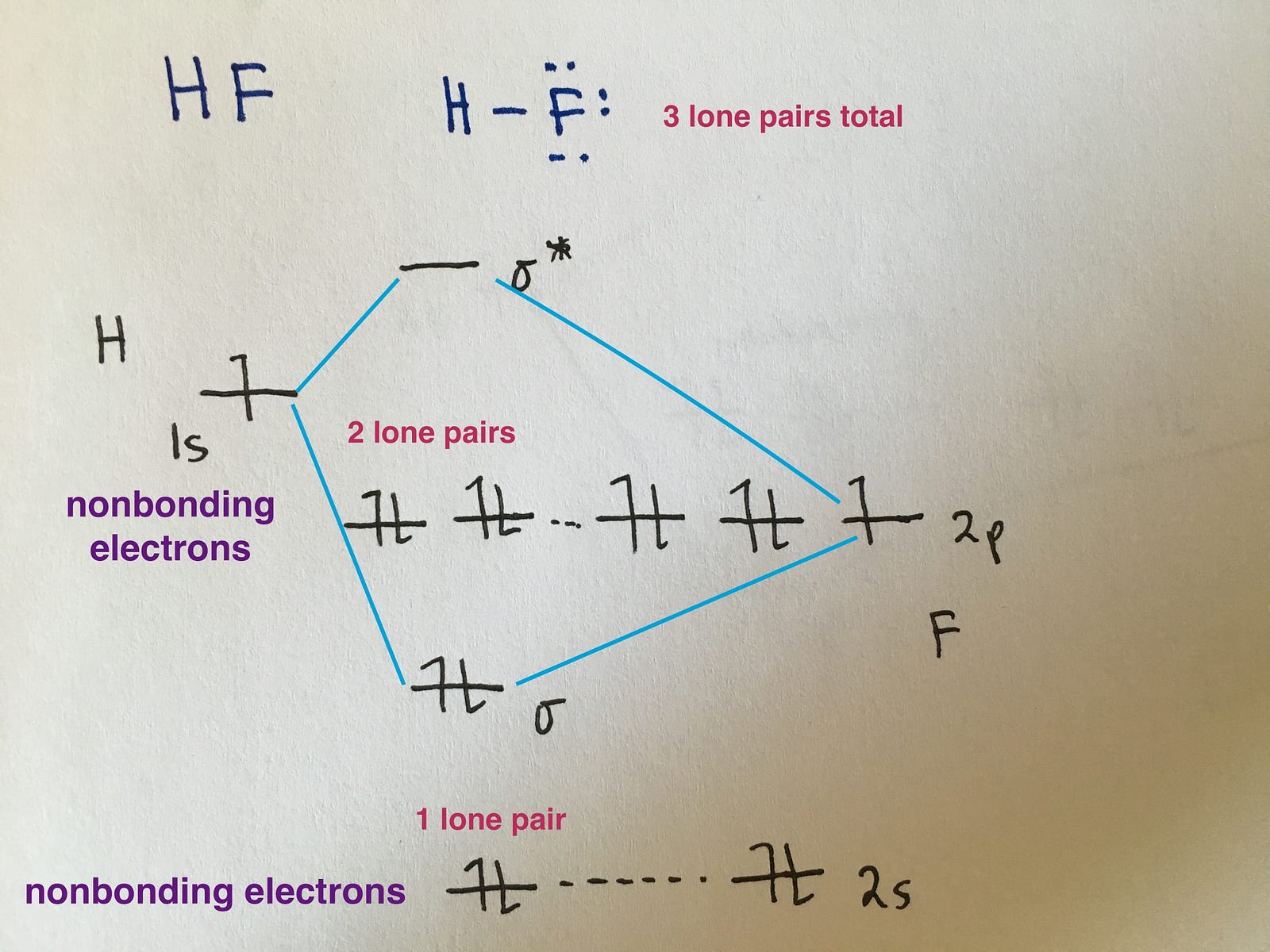
Web however, recall that the more electronegative atom will be lower on the diagram. Web mo diagram for hf. Draw two lines to create three columns. We will predict their bond order and see how the energies of the different orbitals change. Web a class diagram is a uml diagram type that describes a system by visualizing the different types.
MO Diagrams
Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). We will predict their bond order and see how the energies of the different orbitals change. How does this diagram account for the paramagnetism of o 2? We.
MO Diagrams for First Row Diatomic Molecules Chemistry LibreTexts
In the first column, draw the atomic diagram for your first element. Draw two lines to create three columns. How does this diagram account for the paramagnetism of o 2? As atoms bond to form molecules, a certain number of atomic orbitals combine to form. Draw the mo for o 2:
SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
The striker was controversially sent off, following a lengthy var review, in. We need to know what orbitals we are using. Web for more complicated molecules, it is better to use the procedure given earlier: Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding.
MO Diagram Overview, How to draw MO Diagram and Solved example along
Describe the essential difference between a sigma and a pi molecular orbital.; Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. `o_2^+` is just the ionized form of `o_2`; Now let's put these ideas together to make an mo diagram for hf. Determine point group of molecule (if linear, use d.
How To Draw Mo Diagram Wiring Site Resource
To allow space for the next step, draw the. Draw the mo for o 2: We are only going to consider valence orbitals. It also illustrates the operations and attributes of the classes. This approach is used only when the group orbitals are not obvious by inspection.
MO Diagram Overview, How to draw MO Diagram and Solved example along
To allow space for the next step, draw the. In the first column, draw the atomic diagram for your first element. Construct a molecular orbital diagram of the kind shown in this lesson for a simple diatomic molecule, and indicate. Web how to draw molecular orbital diagrams for conjugated systems This approach is used only when the group orbitals are.
how to draw molecular orbital diagrams for polyatomic molecules
Web a class diagram is a uml diagram type that describes a system by visualizing the different types of objects within a system and the kinds of static relationships that exist among them. From this diagram, calculate the bond order for o 2. We need to know what orbitals we are using. Web for more complicated molecules, it is better.
9.8 Molecular Orbital Theory Chemistry Libretexts 22B
They are usually used to explore domain concepts, understand software requirements and. The best way to learn how to draw mo diagrams is to work on practice problems. We are only going to consider valence orbitals. The striker was controversially sent off, following a lengthy var review, in. Draw the mo diagram for `o_2^+` this is a bit of a.
N* = # Electrons In Antibonding Orbitals.
Compare the bond order to that seen in the lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Draw the mo for o 2: As atoms bond to form molecules, a certain number of atomic orbitals combine to form. Based on the amount of orbital overlap, the relative changes in energy differ going from the atomic orbital to the molecular orbital.
We Will Also Compare Our Predictions To Experimental Evidence.
Draw the mo diagram for `o_2^+` this is a bit of a curveball, but a perfectly valid problem. Web for more complicated molecules, it is better to use the procedure given earlier: Try the following mo's on your own, and then check with the answers provided. Now let's put these ideas together to make an mo diagram for hf.
Web Mo Diagram For Hf.
It also illustrates the operations and attributes of the classes. We will predict their bond order and see how the energies of the different orbitals change. We are only going to consider valence orbitals. `o_2^+` is just the ionized form of `o_2`;
Make Sure You Thoroughly Understand The Following Essential Ideas.
Web how to draw molecular orbital diagrams for conjugated systems Define bond order, and state its significance.; H has a 1s orbital. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2 +.