How To Draw Atomic Orbitals
How To Draw Atomic Orbitals - Get a 10 bullets summary of the topic. With oxygen, you know that the atomic orbital potential energies go in the following order: We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. More nodes = more energetic = higher mos. I will use oxygen ( o2(g)) as an example. Web the science classroom. It discusses how to determine the. All orbitals with values of n > 1 and ell = 0 contain one or more nodes. Web since the original \(\psi _{p_{+1}}\) and \(\psi _{p_{+1}}\) were both solutions of the schrödinger wave equation, their combinations are also solutions, and so we can visualize atomic orbitals as shapes along the x,y,z axes. Web bohr's atomic model and its limitations (de broglie's equation, heisenberg’s uncertainty principle), concept of shells, subshells, orbitals.
Orbitals with ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. Web fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. You will also learn how to use hund'. 366k views 10 years ago chemistry tutorials: They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom. With oxygen, you know that the atomic orbital potential energies go in the following order: This knowledge is a precursor to chemical bonding. Web atomic orbitals are the wavefunctions which are solutions of the schroumldinger equation for the hydrogen atom the subset of atomic orbitals and are plotted in three dimensions to exhibit their characteristic shapes the orbitals are drawn by showing their boundary surfaces in the second view and signs are attached to the.
The # of atomic orbitals = the # of conjugated atoms. V 1s << v 2s < v 2p. Keep in mind the energy of the atomic orbitals and molecular orbitals! We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. 1.9m views 3 years ago new ap & general chemistry video playlist. All orbitals with values of n > 1 and ell = 0 contain one or more nodes. 366k views 10 years ago chemistry tutorials: Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. So the atomic orbital diagram is simply those orbitals in that order of energy. By the end of this section, you will be able to:
How to Draw Shapes of Orbitals
This is a way of showing the electron configuration of the atom. Orbitals can be represented as boxes with the electrons depicted with arrows. Web the science classroom. The use of quantum theory provides the best understanding to these topics. Web so, how do these mathematically defined orbitals fit in with the electron shells we saw in the bohr model?
Atomic orbitals explained polizhuge
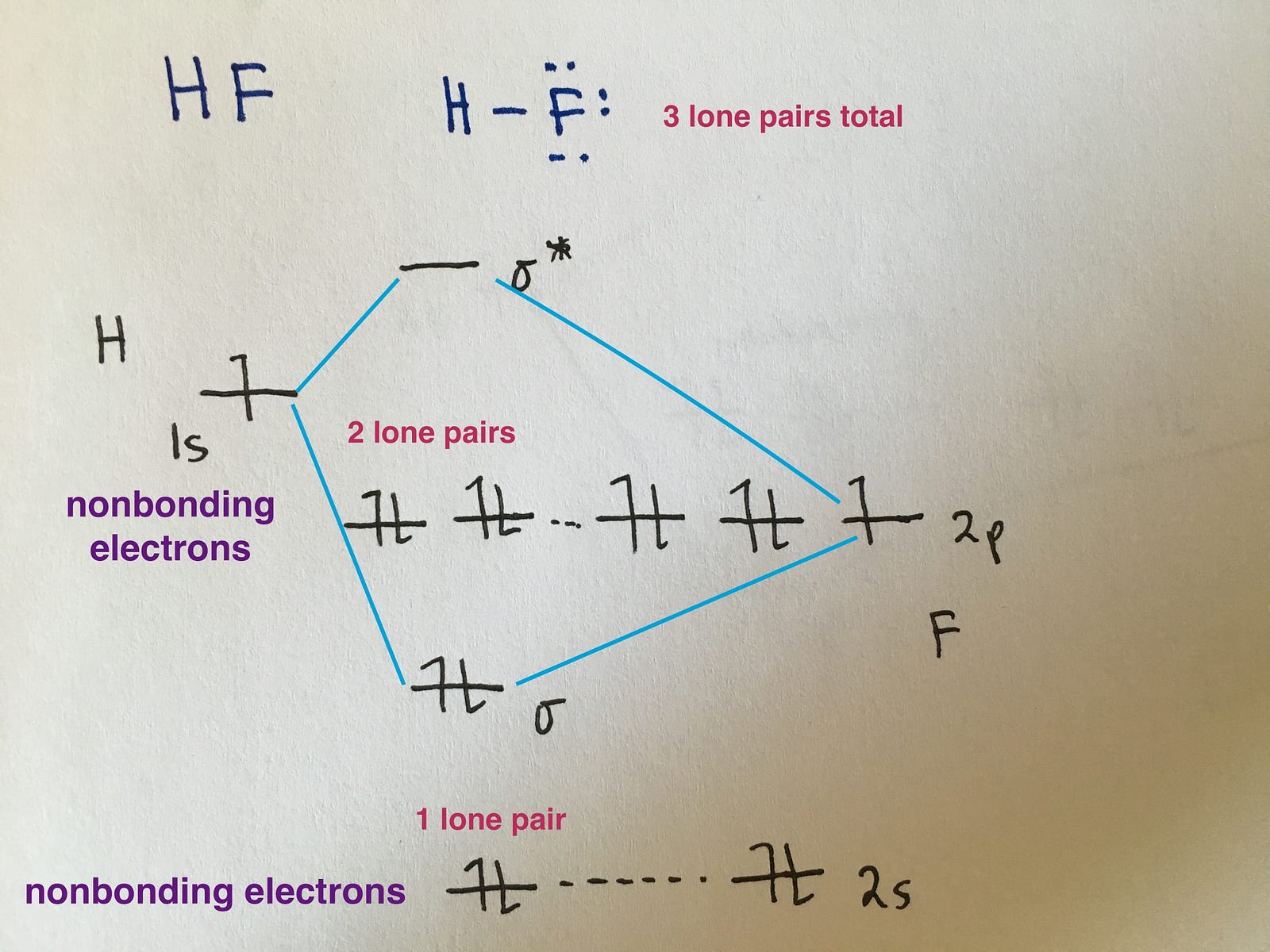
Orbitals can be represented as boxes with the electrons in them shown as arrows. For example, the orbital diagram of li can be shown as: Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. The shape of p orbitals. Quantum numbers, shapes of s,.
How To Draw Orbitals Deepcontrol3
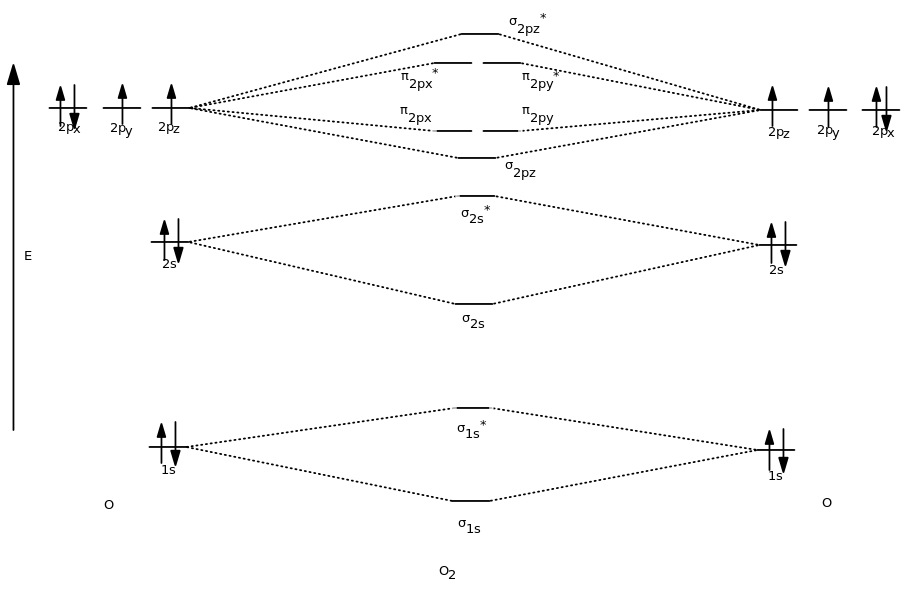
The # of atomic orbitals = the # of conjugated atoms. Web how might one draw atomic and molecular orbital diagrams? Calculate bond orders based on molecular electron configurations. This video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. O2 = (8 − 4) 2 = 2 o 2 = ( 8.
Drawing Atomic and Molecular Orbitals Diagrams for Molecules Organic
I will use oxygen ( o2(g)) as an example. Web figure 3.5.14 3.5. Web so, how do these mathematically defined orbitals fit in with the electron shells we saw in the bohr model? Let's learn about the shapes of atomic orbitals. 2.8k views 8 months ago physical chemistry.
Different Types Of Orbitals
By the end of this section, you will be able to: 366k views 10 years ago chemistry tutorials: All orbitals with values of n > 1 and ell = 0 contain one or more nodes. The following factors contribute to the position of one mo with respect to other mos. Web orbital diagrams use the same basic format, but instead.
Draw The Molecular Orbital Diagram For The Formation Class 11 Chemistry
We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. The molecular orbital energy diagram for o 2 predicts two unpaired electrons. Web bohr's atomic model and its limitations (de broglie's equation, heisenberg’s uncertainty principle), concept of shells, subshells, orbitals. The # of atomic orbitals = the # of.
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
You will also learn how to use hund'. The # of atomic orbitals = the # of conjugated atoms. Subshells are designated by the letters s , p , d , and f , and each letter indicates a different shape. Orbitals can be represented as boxes with the electrons in them shown as arrows. Oxygen's.
Shapes of Atomic Orbitals — Overview & Examples Expii
We calculate the bond order as. Web figure 3.5.14 3.5. Web fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. Get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. You will also learn how to use hund'.
Molecular Orbital Diagrams simplified Megan Lim Medium
The molecular orbital energy diagram for o 2 predicts two unpaired electrons. This video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. Web the goal of this section is to understand the electron orbitals (location of electrons in atoms), their different energies, and other properties. So the atomic orbital diagram is simply.
How To Draw Orbitals Deepcontrol3
Web bohr's atomic model and its limitations (de broglie's equation, heisenberg’s uncertainty principle), concept of shells, subshells, orbitals. Web figure 3.5.14 3.5. Quantum numbers, shapes of s, p and d orbitals. Calculate bond orders based on molecular electron configurations. Orbitals can be represented as boxes with the electrons in them shown as arrows.
1S 2 2S 2 2P 1.
Let's learn about the shapes of atomic orbitals. Web the science classroom. Web the electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Web atomic orbitals are mathematical functions that describe the wave nature of electrons (or electron pairs) in an atom.
Web About Press Copyright Contact Us Creators Advertise Developers Terms Privacy Policy & Safety How Youtube Works Test New Features Nfl Sunday Ticket Press Copyright.
This is a way of showing the electron configuration of the atom. With oxygen, you know that the atomic orbital potential energies go in the following order: Calculate bond orders based on molecular electron configurations. Orbitals can be represented as boxes with the electrons in them shown as arrows.
The Following Factors Contribute To The Position Of One Mo With Respect To Other Mos.
We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. It discusses how to determine the. Web for a given atom, the s orbitals also become higher in energy as n increases because of their increased distance from the nucleus. By the end of this section, you will be able to:
Web How Might One Draw Atomic And Molecular Orbital Diagrams?
Web © 2024 google llc. Web on a tight schedule? Electron configurations are expressed through a notation that looks like this: They offer a way to calculate the probability of finding an electron in a specified region around the nucleus of the atom.