How To Draw Hydrogen Bonding
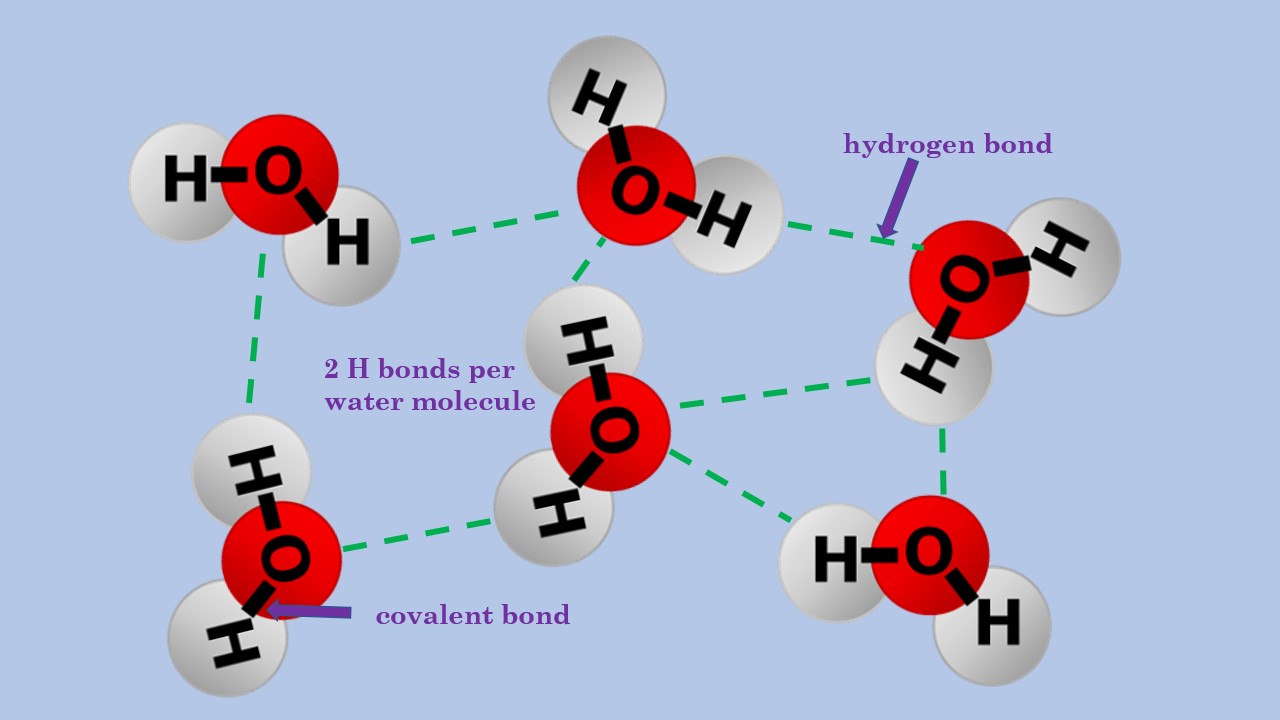
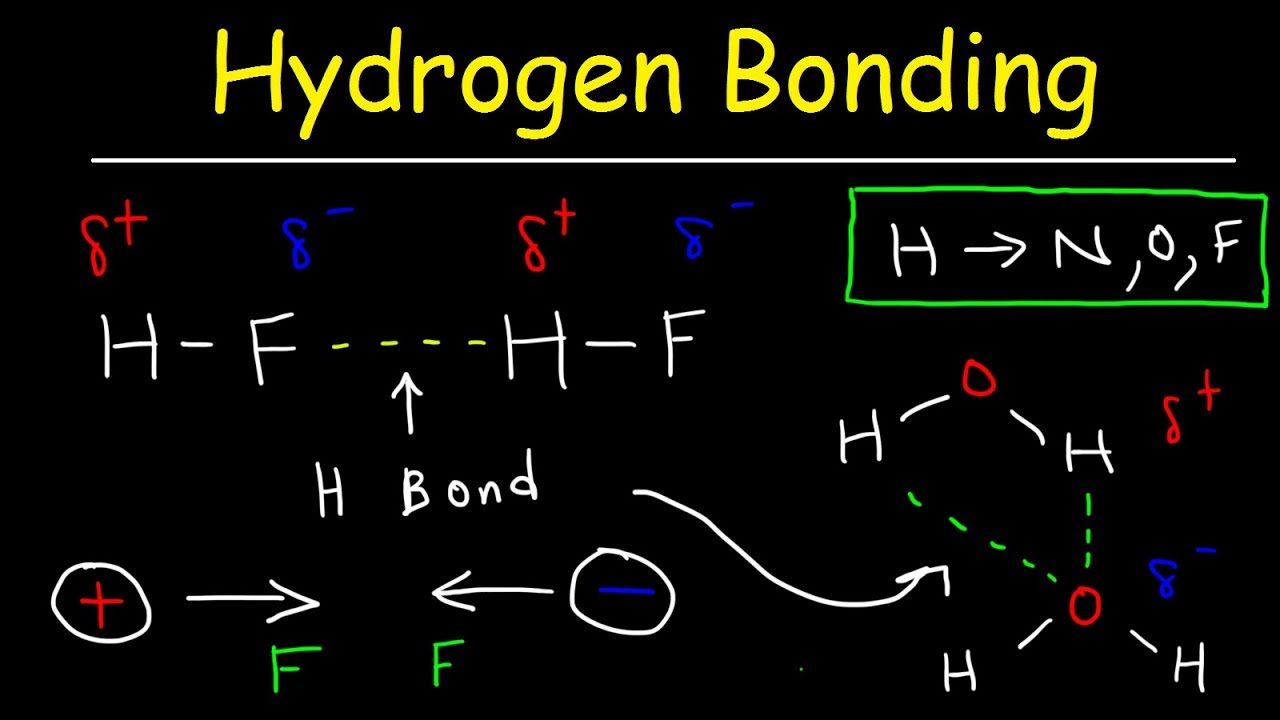
How To Draw Hydrogen Bonding - This video shows three examples of drawing for the formation of hydrogen bond. 10k views 3 years ago #exploding #teacher #chemical. This is why the boiling point of water is higher than. Using lewis structures, we can represent this as follows: The solid line represents a bond in the plane of the screen or paper. If you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Each water molecule is hydrogen bonded to four others. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Hydrogen that is bonded to very electronegative elements (n, o and f) will have a big δ+ (partial positive charge). 4.7k views 7 years ago 1d:
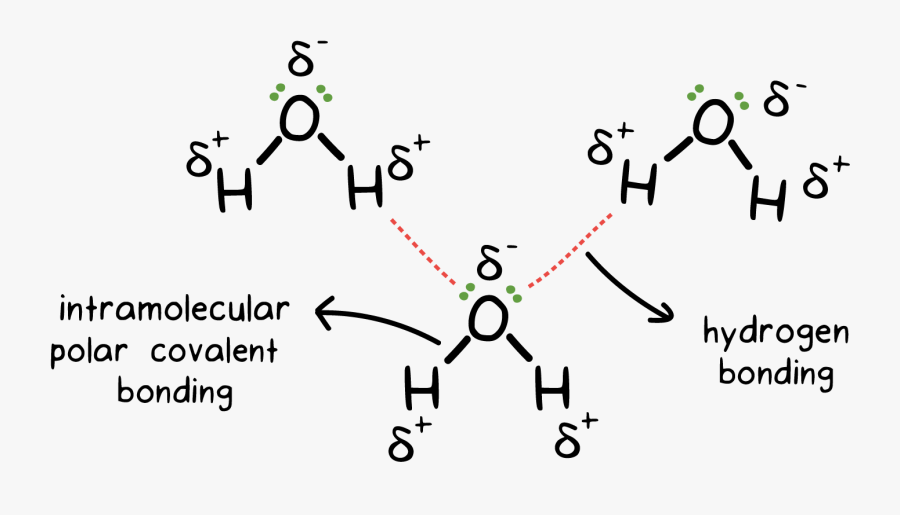
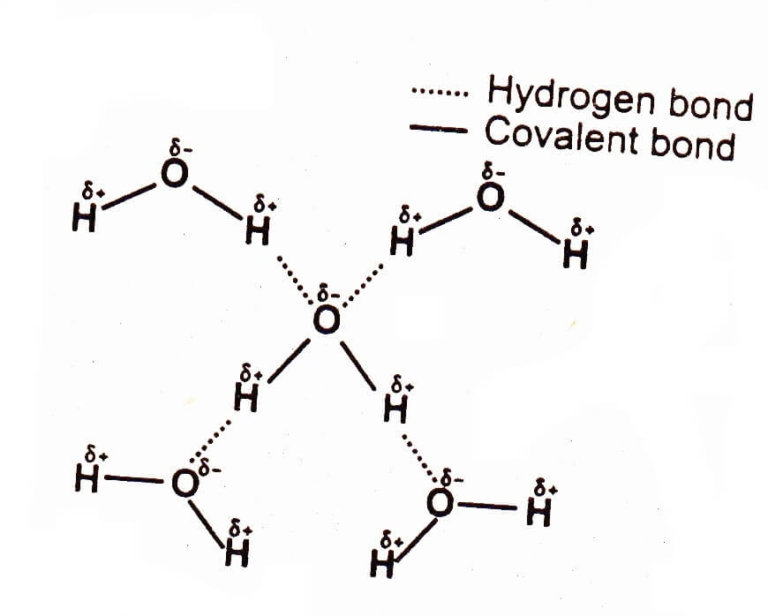
Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Web learn how to study the hydrogen bond in chemical systems using avogadro. Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); The origin of hydrogen bonding. Web hydrogen bonds between water molecules give water its high boiling point, high heat capacity, and surface tension. The number of hydrogen atoms attached to o or n in the molecule. So hydrogen is never a true central atom of a molecule simply because its limited in its bonding abilities. There are various ways of drawing this and you will need to be familiar with all of them. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. 4.7k views 7 years ago 1d:
There are various ways of drawing this and you will need to be familiar with all of them. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Web so, when you're drawing a bond line structure and you have a carbon chain you wanna show that carbon chain in a zig zag pattern. Ammonia can form a maximum of one hydrogen bond per molecule. Next, let's think about the carbon hydrogen bonds. Web exercise \ (\pageindex {1}\) how many hydrogens in figure \ (\pageindex {1}\) can form hydrogen bonds? Does chlorine form hydrogen bonds? Only one, the one at the very top which is attached to the highly electrongative oxygen atom (red), all the others are attached to carbon and can not hydrogen bond. This video shows three examples of drawing for the formation of hydrogen bond. The origin of hydrogen bonding.
Diagram Of Water Molecules Hydrogen Bonding
Each water molecule is hydrogen bonded to four others. Only one, the one at the very top which is attached to the highly electrongative oxygen atom (red), all the others are attached to carbon and can not hydrogen bond. Web for example, two hydrogen atoms can form a bond, producing a molecule of h 2. If you were to draw.
How To Draw Hydrogen Bonds
Note that each atom must contribute one electron to the bond. The origin of hydrogen bonding. Web water molecules forming hydrogen bonds with one another. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Web the force of attraction, shown here as a.
[DIAGRAM] Labeled Diagram Of Hydrogen Bonding
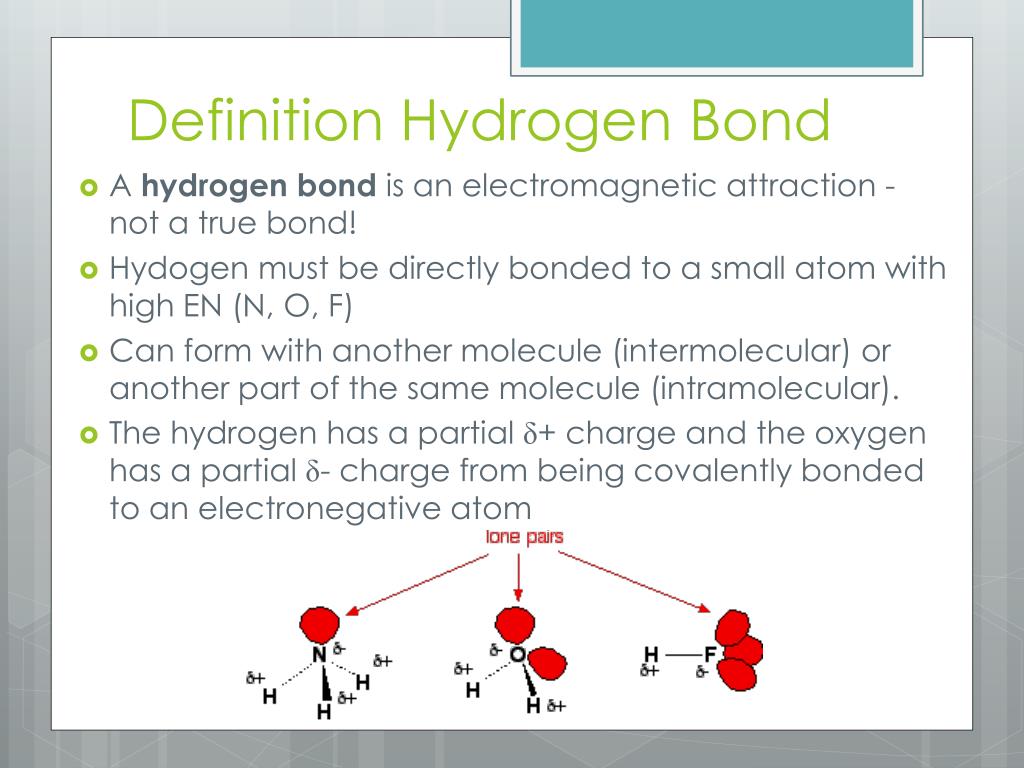
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. The partial negative charge on the o of one molecule can form a hydrogen bond.
Hydrogen Bonding American Chemical Society
In cases like this, the bonding in the organic molecule isn't important. This video shows three examples of drawing for the formation of hydrogen bond. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. Two fluorine atoms can form a molecule of f 2 in the same fashion. The number of.
Draw a diagram of water molecules, labeling the hydrogen bond and
Note that each atom must contribute one electron to the bond. Next, let's think about the carbon hydrogen bonds. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. 4.7k views 7 years ago 1d: This is why the boiling point of water is higher than.
Hydrogen Bonding in water Dr. M. Chemistry Tutor
Atoms can form more than one bond. Web for example, two hydrogen atoms can form a bond, producing a molecule of h 2. Web water as a perfect example of hydrogen bonding. In cases like this, the bonding in the organic molecule isn't important. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams,.
How To Draw Hydrogen Bonds Askworksheet
Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. 4.7k views 7 years ago 1d: Using lewis structures, we can represent this as follows: In cases like this, the bonding in the organic molecule isn't important. Web c5h12 + 8o2 → 5co2 + 6h2o (1) (1) c 5 h 12 + 8 o 2.
H2o Drawing Chemical Bond Intermolecular Hydrogen Bonding In Water
Web the force of attraction, shown here as a dotted line, is called a hydrogen bond. Does chlorine form hydrogen bonds? The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. The molecules which have this extra bonding are: These properties allow cells to.
Hydrogen Bonding Chemistry Skills
The number of lone pairs on the o or n. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Hydrogen bonding between different parts of the same chain (intramolecular bonding; Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. There are various.
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Hydrogen bonding between different parts of the same chain (intramolecular bonding; Web c5h12 + 8o2 → 5co2 + 6h2o (1) (1) c 5 h 12 + 8 o 2 → 5 c o 2 + 6 h 2 o. So hydrogen is never a true central atom of a molecule simply because its limited in its bonding abilities. This is.
Web Hydrogen Bonds Between Water Molecules Give Water Its High Boiling Point, High Heat Capacity, And Surface Tension.
There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. The molecules which have this extra bonding are: Web c5h12 + 8o2 → 5co2 + 6h2o (1) (1) c 5 h 12 + 8 o 2 → 5 c o 2 + 6 h 2 o. If you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever.
Web Exercise \ (\Pageindex {1}\) How Many Hydrogens In Figure \ (\Pageindex {1}\) Can Form Hydrogen Bonds?
In cases like this, the bonding in the organic molecule isn't important. Web these relatively powerful intermolecular forces are described as hydrogen bonds. Ammonia can form a maximum of one hydrogen bond per molecule. This is why the boiling point of water is higher than.
So Hydrogen Is Never A True Central Atom Of A Molecule Simply Because Its Limited In Its Bonding Abilities.
Hydrogen that is bonded to very electronegative elements (n, o and f) will have a big δ+ (partial positive charge). Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); The number of lone pairs on the o or n. Note that each atom must contribute one electron to the bond.
Web Water As A Perfect Example Of Hydrogen Bonding.
The number of hydrogen bonds depends on: 4.7k views 7 years ago 1d: A structural formula shows how the various atoms are bonded. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom.

![[DIAGRAM] Labeled Diagram Of Hydrogen Bonding](http://ffden-2.phys.uaf.edu/webproj/211_fall_2016/Roger_Vang/Roger_Vang/Hydrogen Bonding.png)