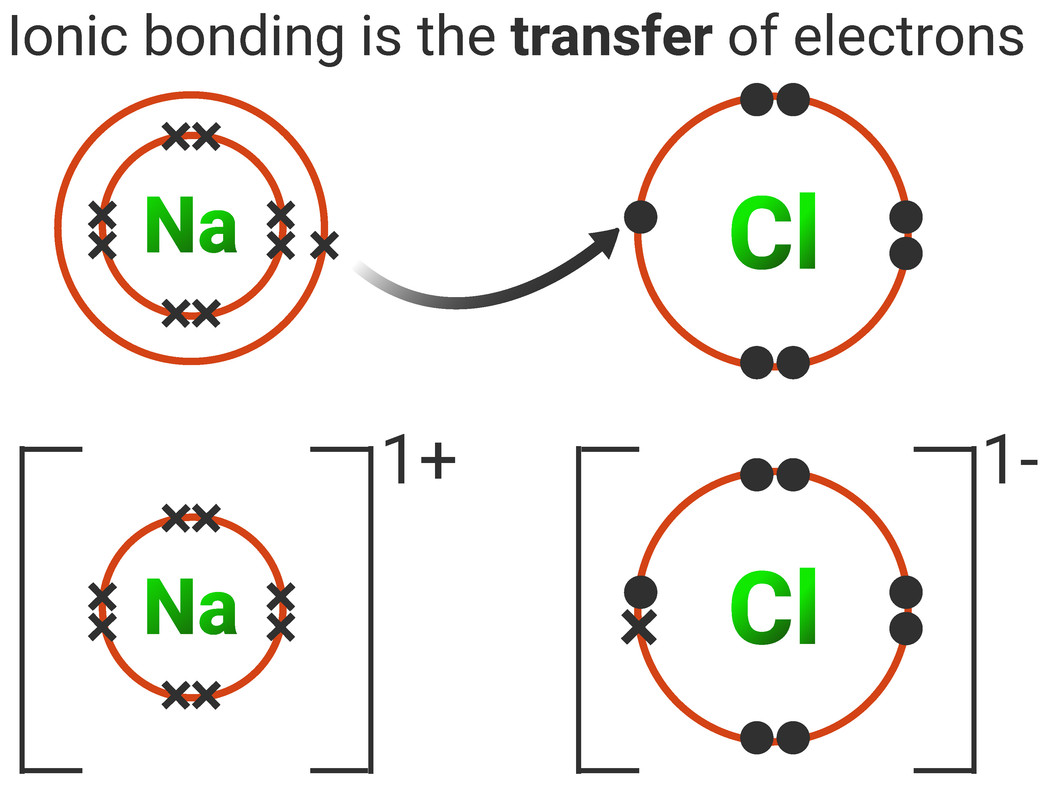
How To Draw Ionic Bonds
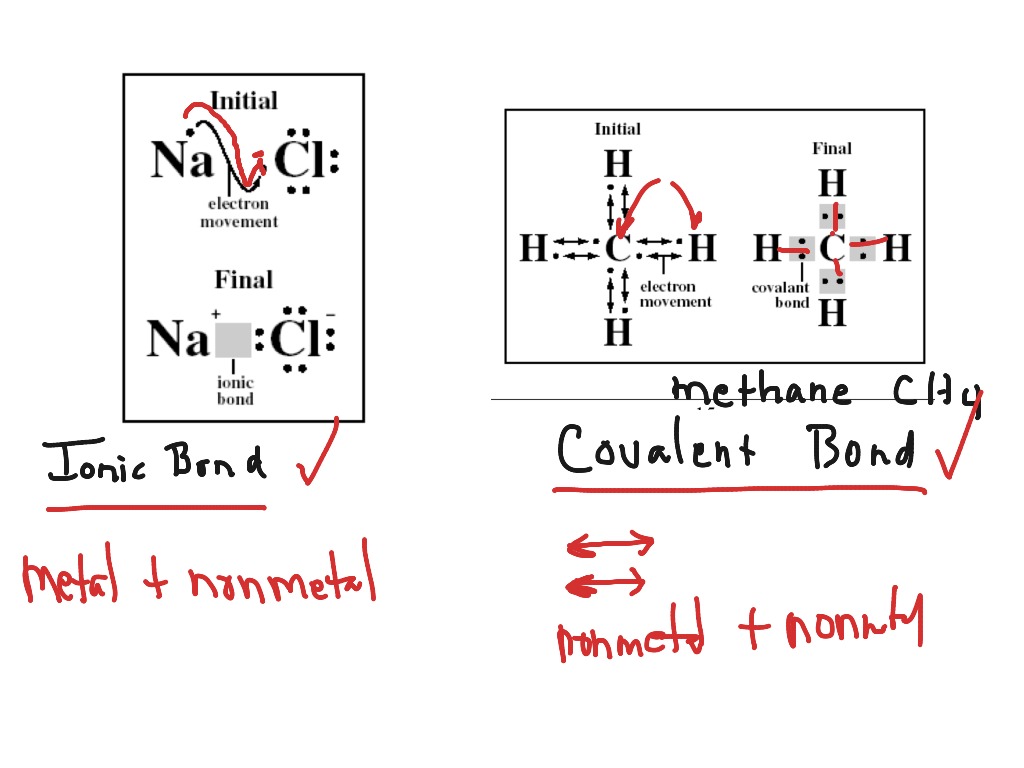
How To Draw Ionic Bonds - The metal atoms become positive ions and. So, ionic bond between only metals is not possible. 6.7k views 7 years ago edexcel. Magnesium has two electrons in its outer shell, oxygen has six. Draw the outer shell of each atom. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Web shows how to draw lewis dot structures for ionic compounds. Lithium + oxygen → lithium oxide. Two metals can't form an ionic bond.
While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. Using lewis symbols for ionic compounds. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. For example, two hydrogen atoms can form a bond, producing a molecule of h 2. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Note that each atom must contribute one electron to the bond. Web ionic bonding mats this resource accompanies the poster how to draw ionic bonds from education in chemistry which can be viewed at rsc.li/3amzz9j learning objectives 1 draw dot and cross diagrams for ionic compounds. 2 show how electrons are transferred in ionic bonding. Once they have mastered electron configuration diagrams, show your learners how they can adapt them to show structure and bonding in covalent and ionic compounds.
Introductory, conceptual, and gob chemistry. Lithium + oxygen → lithium oxide. Web shows how to draw lewis dot structures for ionic compounds. Web © 2023 google llc. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Look at the number of electrons on the outer shell of each atom. These grades are the stepping stone to your future. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. This chemistry video explains how to draw the lewis structures of ionic compounds. Draw the outer shell of each atom.
[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams
Positive and negative ions form when a. Even if you don't want to stud. Ionic bonds require at least one electron donor and one electron acceptor. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. These grades are the stepping stone to your future.
How to draw ionic bonding dot and cross diagrams Feature RSC Education
The requirements for this bond are the losing of electrons by one element and gaining by another. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Draw the electron configuration diagrams for each atom in the compound. It means that you can't state exactly how many.
Ionic Bonds Chemical bond, Ionic bonding, Ionic
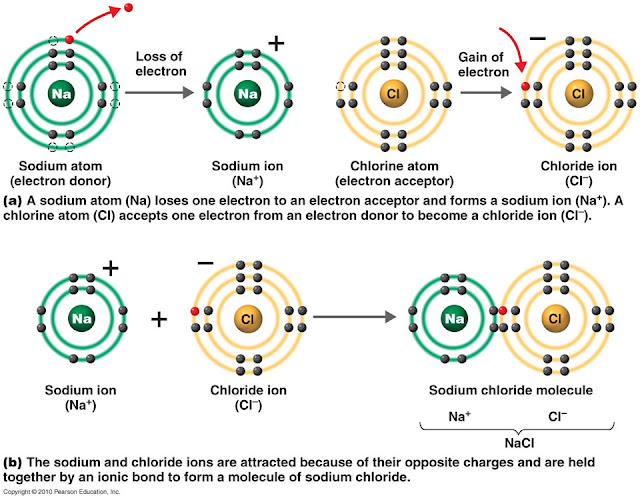
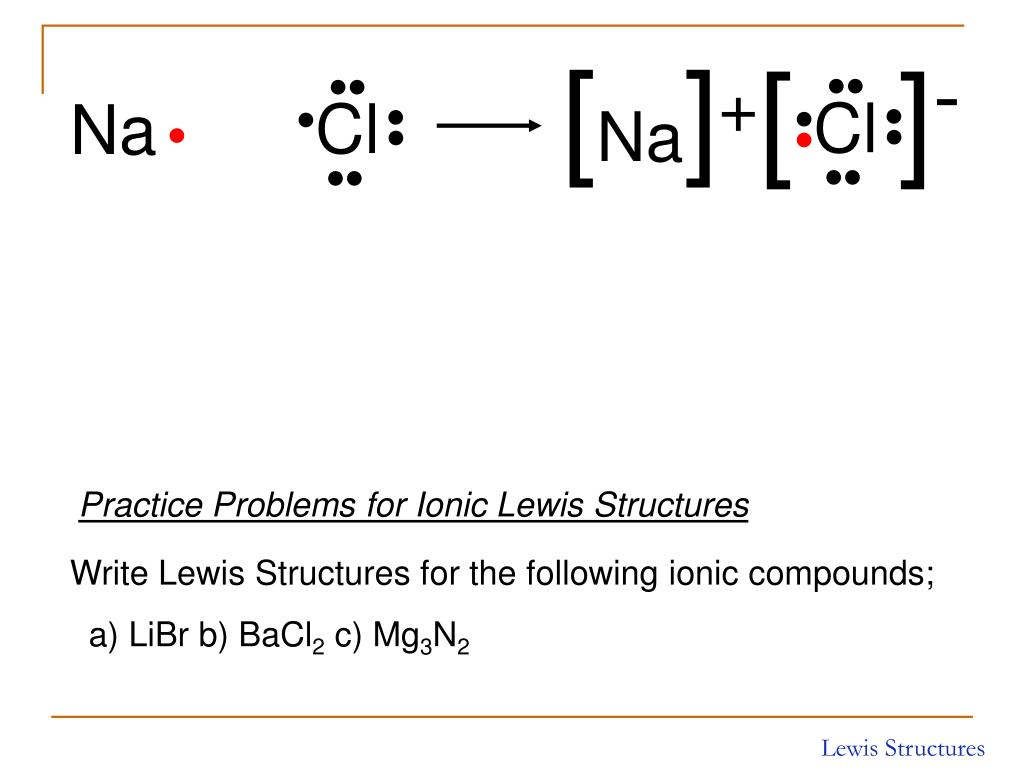
Web calcium + chlorine → calcium chloride. Web draw a lewis electron dot diagram for an atom or a monatomic ion. In chapter 1, we used atomic theory to describe the structure of the fluorine atom. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: For exam.
Lewis Dot Structure Ionic Compounds
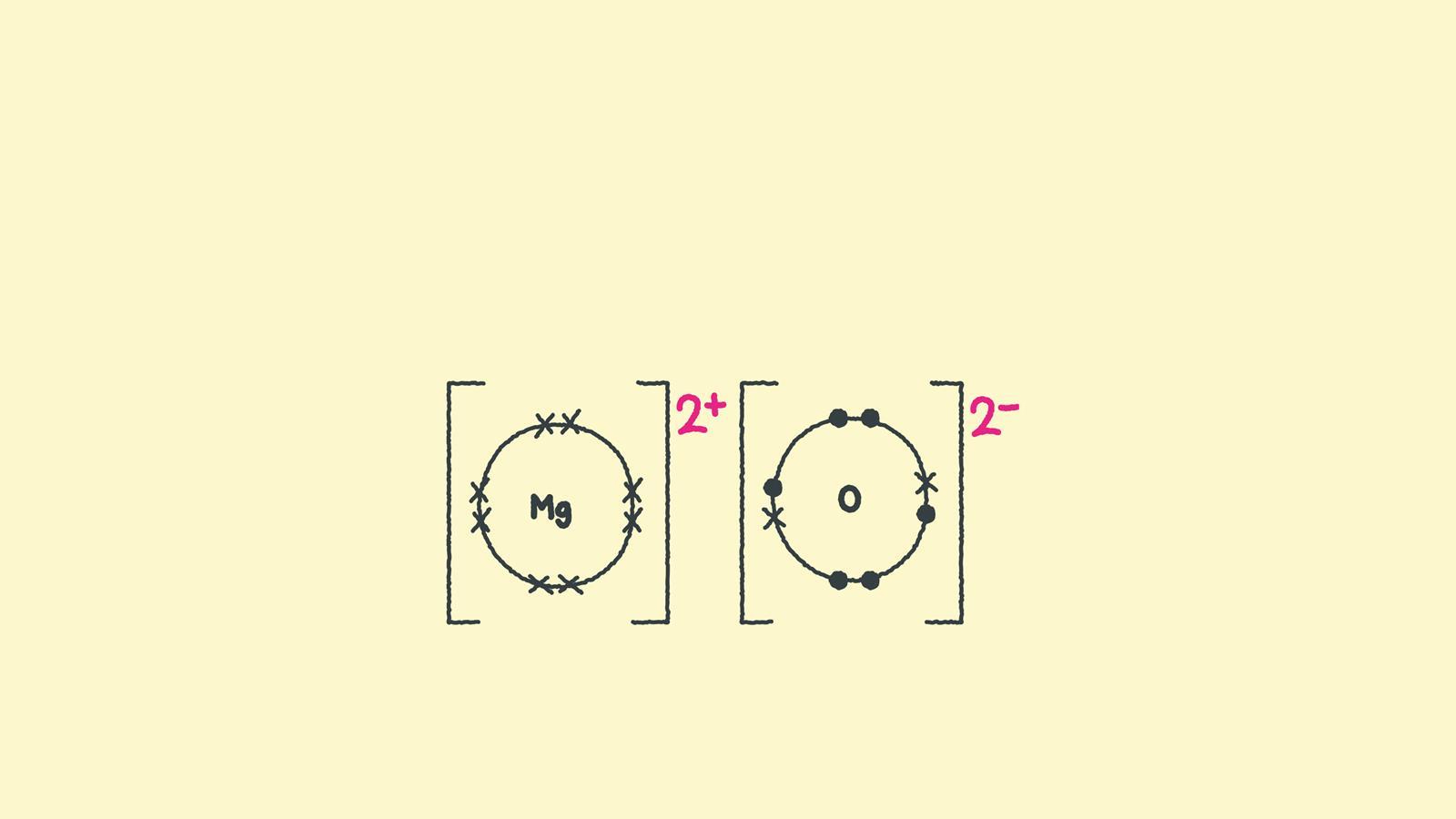
Magnesium oxide dot & cross diagram. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. For example, two hydrogen atoms can form a bond, producing a molecule of h 2. Introductory, conceptual, and gob chemistry. 2 show how electrons are transferred in ionic bonding.
Ionic Bonding Diagram
In chapter 1, we used atomic theory to describe the structure of the fluorine atom. Magnesium oxide dot & cross diagram. For example, two hydrogen atoms can form a bond, producing a molecule of h 2. Web © 2023 google llc. Introductory, conceptual, and gob chemistry.
Ionic Bonding Diagram
Web each atom contributes one electron to the bond. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. You should be clear that giant in this context doesn't just mean very large. Web in ionic.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Positive and negative ions form when a. Rsc.li/2whsi4f if you need a reminder). Magnesium has two electrons in its outer shell, oxygen has six. Web shows how to draw lewis dot structures for ionic compounds. Introductory, conceptual, and gob chemistry.
Drawing Ionic Bonds Worksheet Kid Worksheet Printable
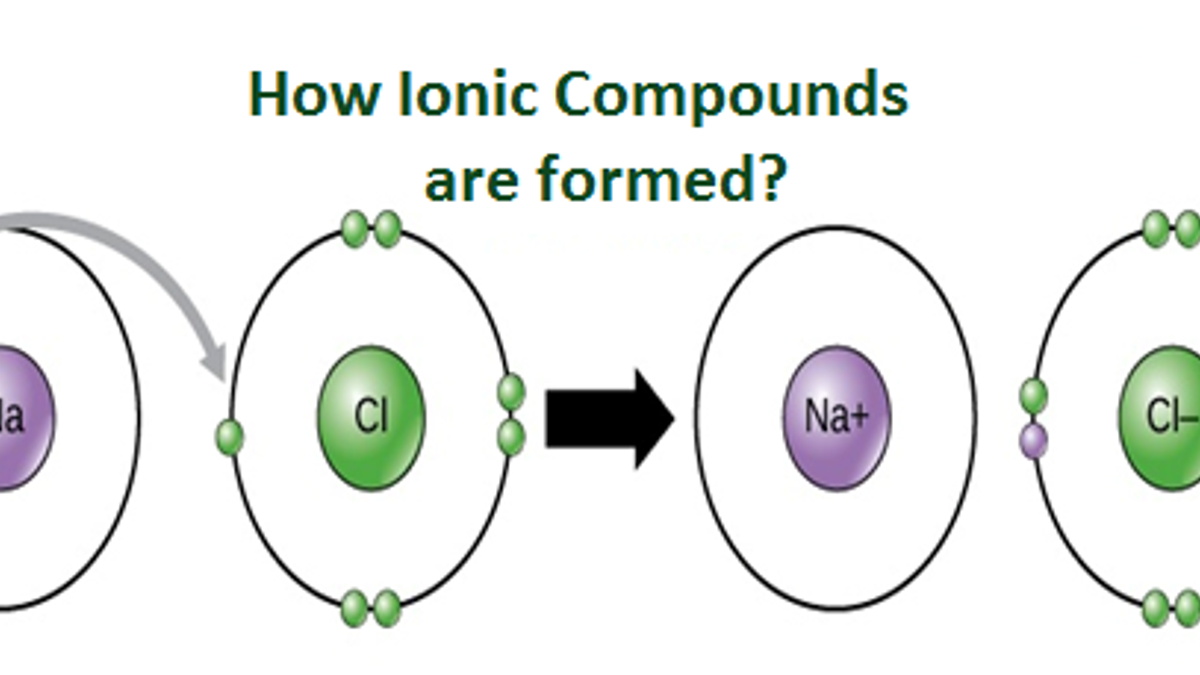
For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Web in ionic bonding, atoms transfer electrons to each other. Web the two types of bonding are covalent, for the sharing of electrons between atoms, and ionic,.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Swap the crosses for dots in one of your diagrams. Positive and negative ions form when a. Lithium + oxygen → lithium oxide. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Web the two types of bonding are covalent, for the sharing of electrons between.
Lewis Structure Of Ionic Compounds
Using lewis structures, we can represent this as follows: Web each atom contributes one electron to the bond. Web the two types of bonding are covalent, for the sharing of electrons between atoms, and ionic, for the net transfer of electrons between atoms. Using lewis symbols for ionic compounds. Web shows how to draw lewis dot structures for ionic compounds.
Web The Two Types Of Bonding Are Covalent, For The Sharing Of Electrons Between Atoms, And Ionic, For The Net Transfer Of Electrons Between Atoms.
Web the attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Instead ionic compounds stick together through electrostatic forces (different electrically charged ions) which we usually represent with brackets and the charge in the upper right corner. You should be clear that giant in this context doesn't just mean very large. Even if you don't want to stud.
Using Lewis Structures, We Can Represent This As Follows:
The metal atoms become positive ions and. Web © 2023 google llc. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Magnesium has two electrons in its outer shell, oxygen has six.
Two Fluorine Atoms Can Form A Molecule Of F 2 In The Same Fashion.
Positive and negative ions form when a. Web each atom contributes one electron to the bond. Two metals can't form an ionic bond. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell.
When Drawing Lewis Dot Structures For Ionic Compounds You Need To Follow A Different Set Of Rules Than With Lewis Structures For Covalent/Molecular Compounds.
Draw the outer shell of each atom. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: Web in ionic bonding, atoms transfer electrons to each other. Chemistry for changing times (hill and mccreary) 4:
![[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams](https://showme0-9071.kxcdn.com/files/27469/pictures/thumbs/1481193/last_thumb1396045740.jpg)