How To Draw Proline In A Peptide Chain
How To Draw Proline In A Peptide Chain - Although drawn as a single bond, the peptide bond behaves more like a double bond, or rather like a bond and a half. Amino acids are the building blocks of proteins. Proline’s presence in a protein affects its secondary structure. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Web proline is a unique amino acid due to the covalent bond between the backbone nitrogen and the proline side chain. Web peptide bond rotation and proline: Many biologically important peptide sequences contain proline. Glycine can cause a bend in the chain, because it has extreme conformation mobility, due to its small size. Web the resulting link between the amino acids is an amide link which biochemists call a peptide bond. Web because proline has an odd, cyclic structure, when it forms peptide bonds, it induces a bend into the amino acid chain.
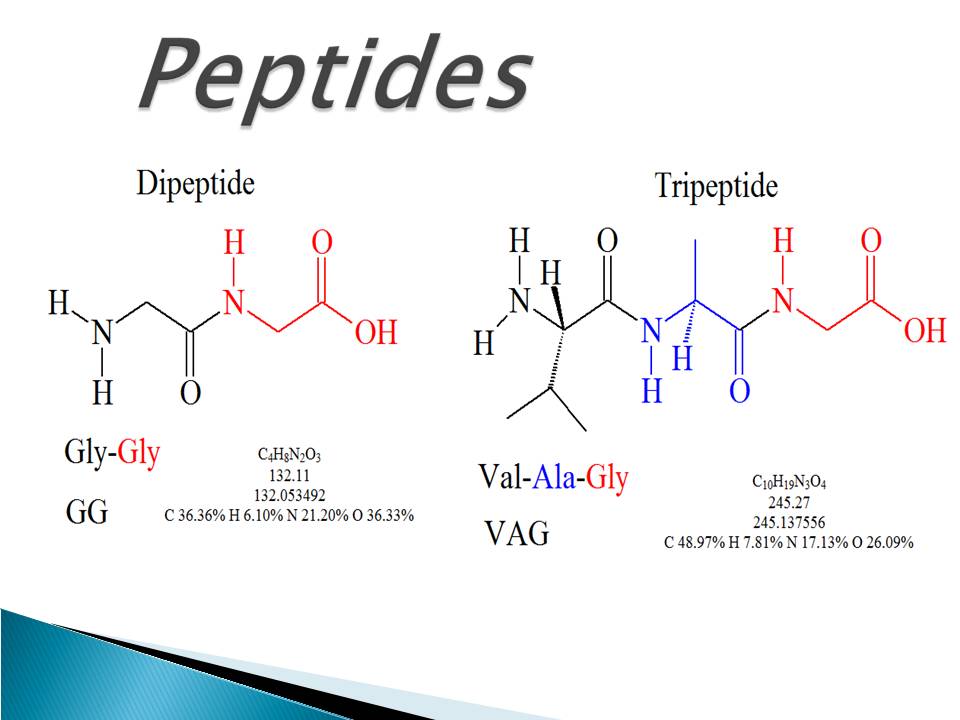
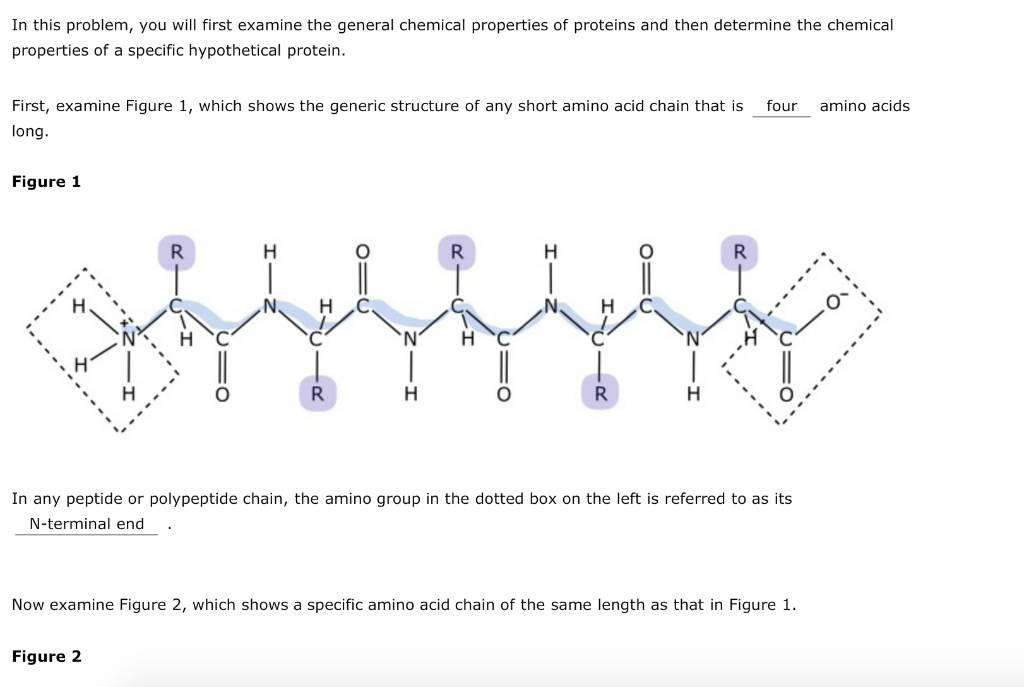
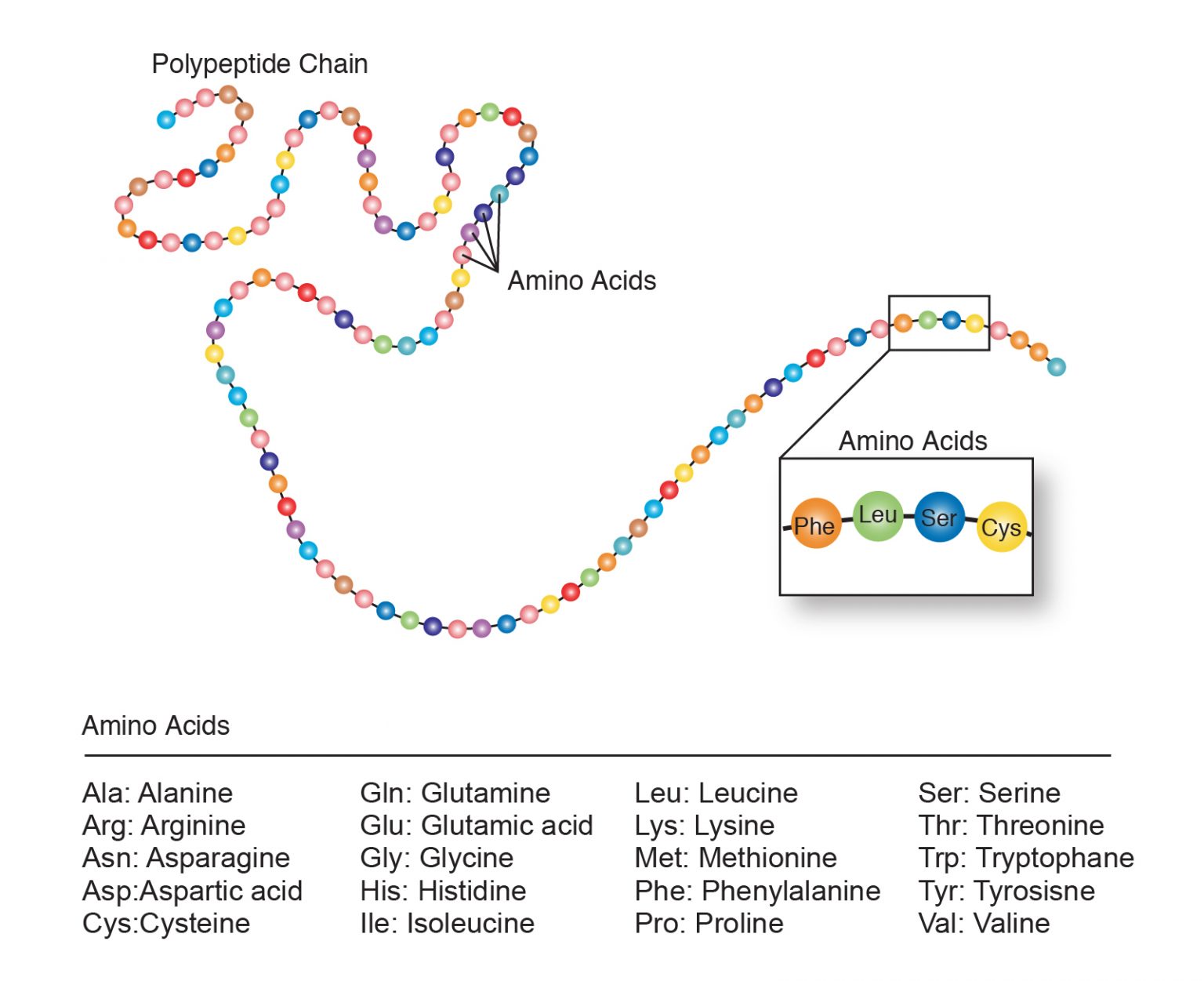
Peptides are functioning in human body on many ways, such as regulating metabolism (insulin) and mediating pain signals (dynorphin). Web a tool that draws peptide primary structure and calculates theoretical peptide properties. Amino acids are the building blocks of proteins. In this reaction, water is released. When two amino acids link together to form an amide link, the resulting structure is called a dipeptide. Web proline is a unique amino acid due to the covalent bond between the backbone nitrogen and the proline side chain. They also play a role in endocrine signaling and can act as a growth factor. The only exception to this rule is proline, which has no hydrogens attached to its nitrogen since it is already connected to its own functional group. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Web the unique sequence of amino acids in a polypeptide chain is its primary structure.
Many biologically important peptide sequences contain proline. Web peptide bonds are the vital links that connect amino acids to form polypeptide chains, which fold into functional proteins. The only exception to this rule is proline, which has no hydrogens attached to its nitrogen since it is already connected to its own functional group. Web because proline has an odd, cyclic structure, when it forms peptide bonds, it induces a bend into the amino acid chain. Structurally, proline (figure 6) is unique among the aas because its side chain loops around and reconnects with the peptide backbone. These proteins are also called polypeptides. In a reverse reaction, the peptide bond can be cleaved by water (hydrolysis). It is the least flexible of the protein amino acids and thus gives conformational rigidity when present in a protein. In the case of a single bond, there is free rotation around the bond axis in response to molecular collisions. Proline’s presence in a protein affects its secondary structure.
Peptide Bond Definition, Formation, Structure, Examples
Peptides are functioning in human body on many ways, such as regulating metabolism (insulin) and mediating pain signals (dynorphin). Web the unique sequence of amino acids in a polypeptide chain is its primary structure. Web many proteins are formed by not only one strand of amino acids, but many. Structurally, proline (figure 6) is unique among the aas because its.
LabXchange
Most amino acids have a chiral carbon, which allows them to rotate polarized light. [google scholar] okabayashi h, isemura t, sakakibara s. In the case of a single bond, there is free rotation around the bond axis in response to molecular collisions. Web proline is a unique amino acid due to the covalent bond between the backbone nitrogen and the.
Amino Acid Polypeptide Chain Structure CH103 Chapter 8 The Major
Understand what proline is and how it differs from other amino acids. Web a chain consisting of only two amino acid units is called a dipeptide; They also play a role in endocrine signaling and can act as a growth factor. Many biologically important peptide sequences contain proline. Web many biologically important peptide sequences contain proline.
Amino acids physical, chemical properties and peptide bond
I challenge you to draw the peptide chain with proline; Peptides are functioning in human body on many ways, such as regulating metabolism (insulin) and mediating pain signals (dynorphin). Web a chain consisting of only two amino acid units is called a dipeptide; They contain an amino group, carboxylic acid group, alpha carbon, and side chain. Glycine can cause a.
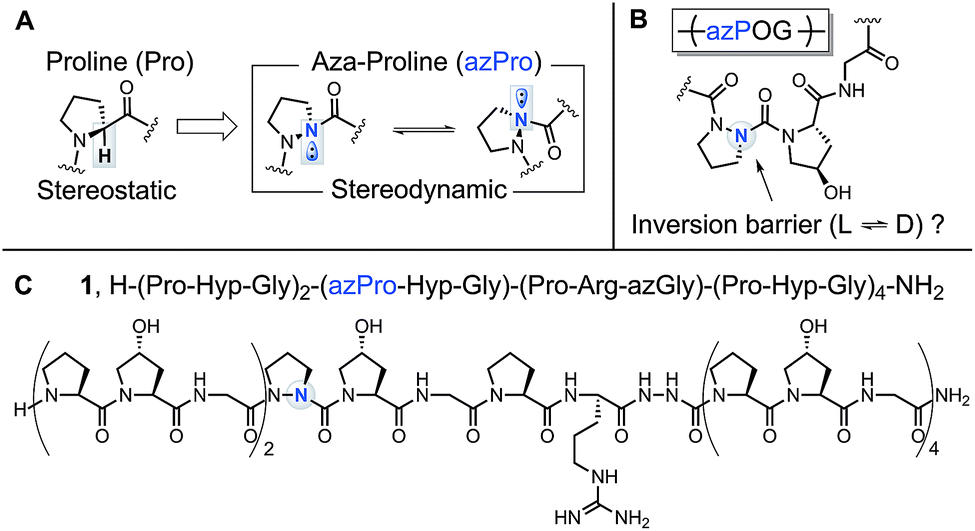
Azaproline effectively mimics l proline stereochemistry in triple
Web many proteins are formed by not only one strand of amino acids, but many. But since most proteins are not only composed of one chain you cannot call them a peptide, but a polypeptide. Web peptide bond formation mechanism. This page was last updated: [google scholar] okabayashi h, isemura t, sakakibara s.
Proline Chemical Structure. Vector Illustration Hand Drawn Stock Vector
The only exception to this rule is proline, which has no hydrogens attached to its nitrogen since it is already connected to its own functional group. Figure 6.proline (top) and glycine (bottom). Web the unique sequence of amino acids in a polypeptide chain is its primary structure. They contain an amino group, carboxylic acid group, alpha carbon, and side chain..
What Is A Polypeptide Chain Definition Types Bond And Examples Images
Glycine can cause a bend in the chain, because it has extreme conformation mobility, due to its small size. Understand what proline is and how it differs from other amino acids. Web the unique sequence of amino acids in a polypeptide chain is its primary structure. Web many biologically important peptide sequences contain proline. I challenge you to draw the.
Figure S3 Schematic drawing of ( + H 2 NHydroxyprolineProline
Web many proteins are formed by not only one strand of amino acids, but many. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Understand what proline is and how it differs from other amino acids. Web peptide bond rotation and proline: The primary structure is coded for in the dna, a process you will.
Protein Structure and Function An Interactive Introduction to
The primary structure is coded for in the dna, a process you will learn about in the transcription and translation. This tool allows to construct peptide sequence and calculate molecular weight and molecular formula. Web peptides often contain up to fifty amino acid residues, protein are molecules with more than fifty amino acid residues. The only exception to this rule.
Introduction to proteins in molecular biology GoldBio
Web peptide bonds are the vital links that connect amino acids to form polypeptide chains, which fold into functional proteins. Amino acids are the building blocks of proteins. Web proline is a unique amino acid due to the covalent bond between the backbone nitrogen and the proline side chain. Web peptides often contain up to fifty amino acid residues, protein.
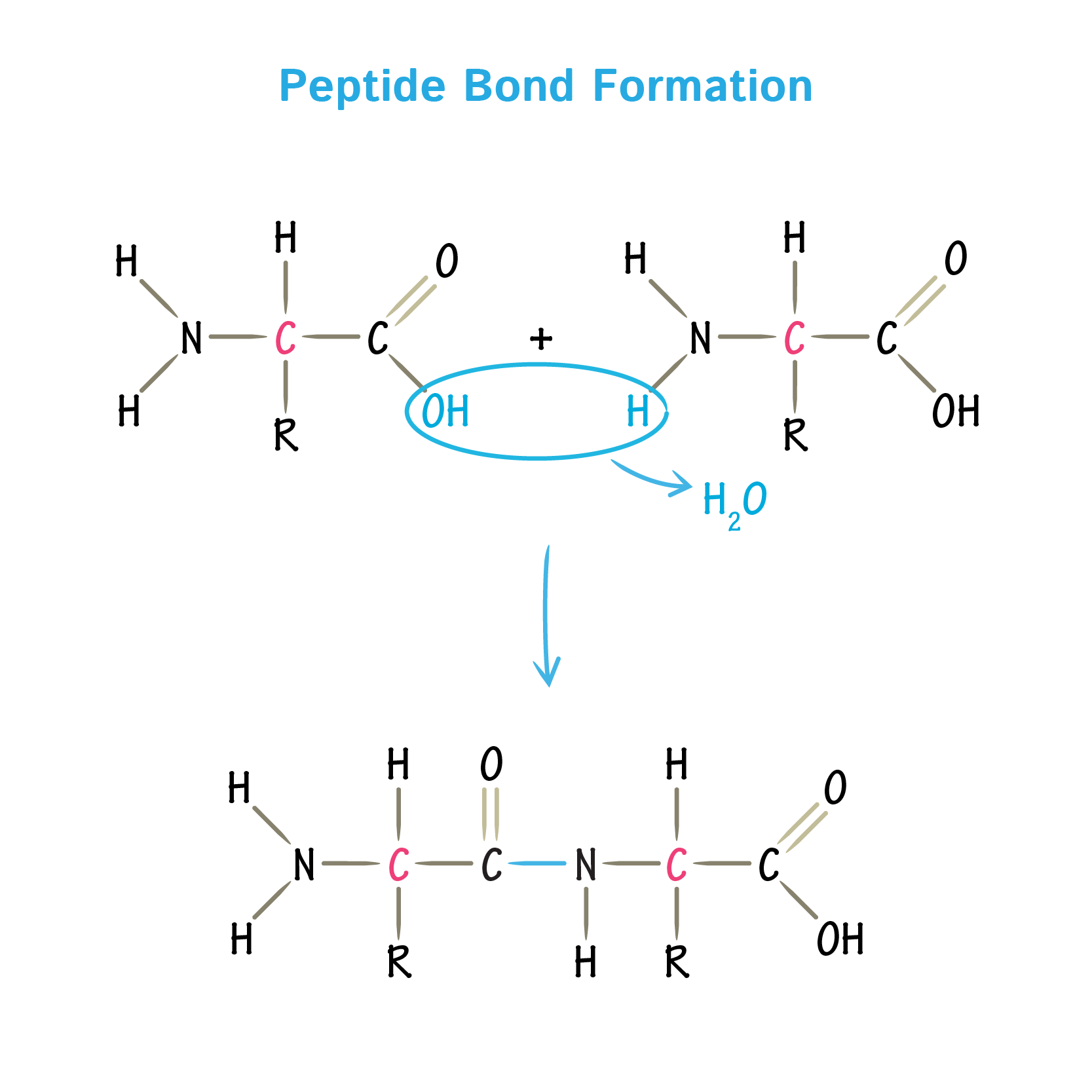
When Two Amino Acids Link Together To Form An Amide Link, The Resulting Structure Is Called A Dipeptide.
In this reaction, water is released. Most amino acids have a chiral carbon, which allows them to rotate polarized light. [google scholar] okabayashi h, isemura t, sakakibara s. Web many biologically important peptide sequences contain proline.
The Only Exception To This Rule Is Proline, Which Has No Hydrogens Attached To Its Nitrogen Since It Is Already Connected To Its Own Functional Group.
Although drawn as a single bond, the peptide bond behaves more like a double bond, or rather like a bond and a half. This page was last updated: Glycine can cause a bend in the chain, because it has extreme conformation mobility, due to its small size. Figure 6.proline (top) and glycine (bottom).
Web Peptides Often Contain Up To Fifty Amino Acid Residues, Protein Are Molecules With More Than Fifty Amino Acid Residues.
Web many proteins are formed by not only one strand of amino acids, but many. Web proline and glycine. But since most proteins are not only composed of one chain you cannot call them a peptide, but a polypeptide. Int j pept protein res.
In A Reverse Reaction, The Peptide Bond Can Be Cleaved By Water (Hydrolysis).
Web proline is a unique amino acid due to the covalent bond between the backbone nitrogen and the proline side chain. They contain an amino group, carboxylic acid group, alpha carbon, and side chain. The primary structure is coded for in the dna, a process you will learn about in the transcription and translation. Structurally, proline (figure 6) is unique among the aas because its side chain loops around and reconnects with the peptide backbone.