How To Read A Solubility Curve
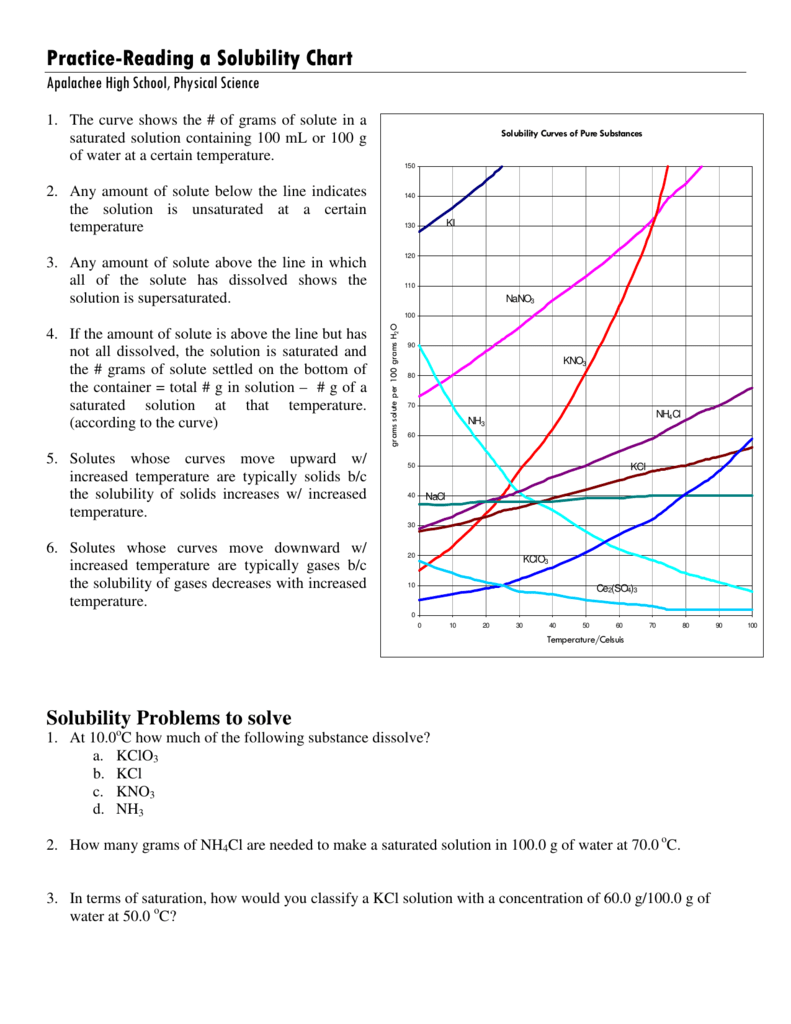
How To Read A Solubility Curve - Solubility curves for more than one substance are often drawn on the same. The solubility curve line shows you with a saturated solution. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web how to read the solubility curve?
Web how to read the solubility curve? Solubility curves for more than one substance are often drawn on the same. The solubility curve line shows you with a saturated solution. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c.
Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. The solubility curve line shows you with a saturated solution. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Solubility curves for more than one substance are often drawn on the same. Web how to read the solubility curve?
Reading Solubility Graphs YouTube
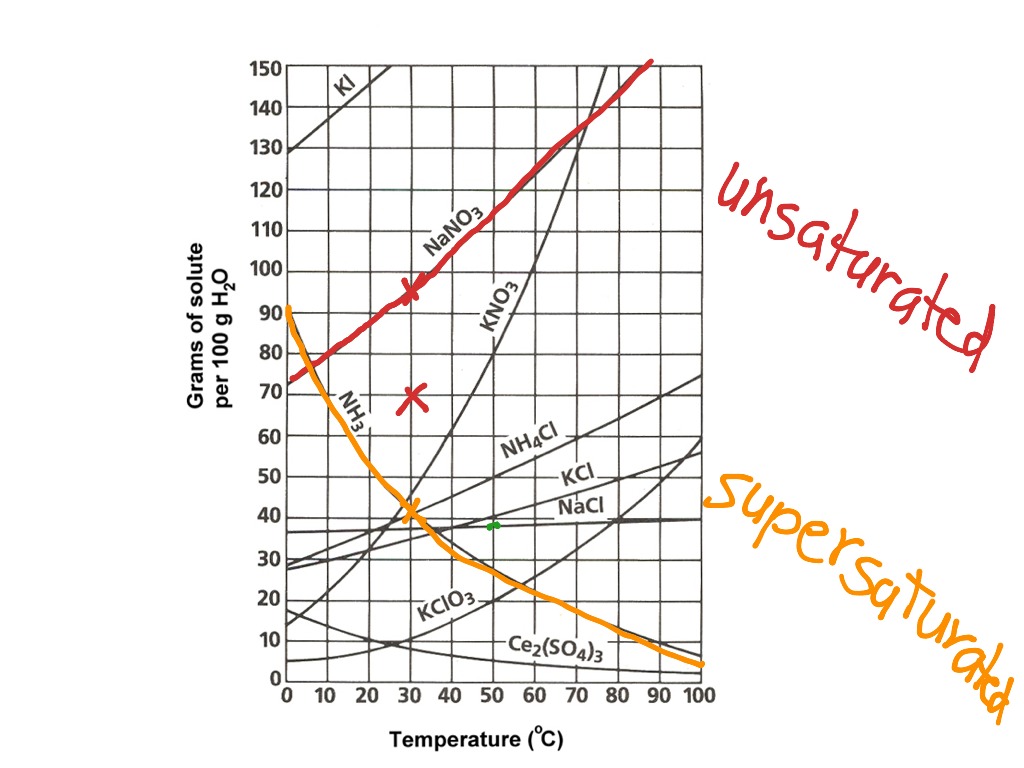
Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. The solubility curve line shows you with a saturated solution. Web how to read the solubility curve? A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web since a solubility curve only shows you.
Reading a SolubilityChart.doc Google Docs
Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. The solubility curve line shows you with a saturated solution. Web how to read the solubility curve? Solubility curves for more than one substance are often drawn on the same. Web since a solubility curve only shows you the amount of solute.
PracticeReading a Solubility Chart
Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Solubility curves for more than one substance are often drawn on the same. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web since a solubility curve only shows you the amount of solute.
ShowMe solubility curve
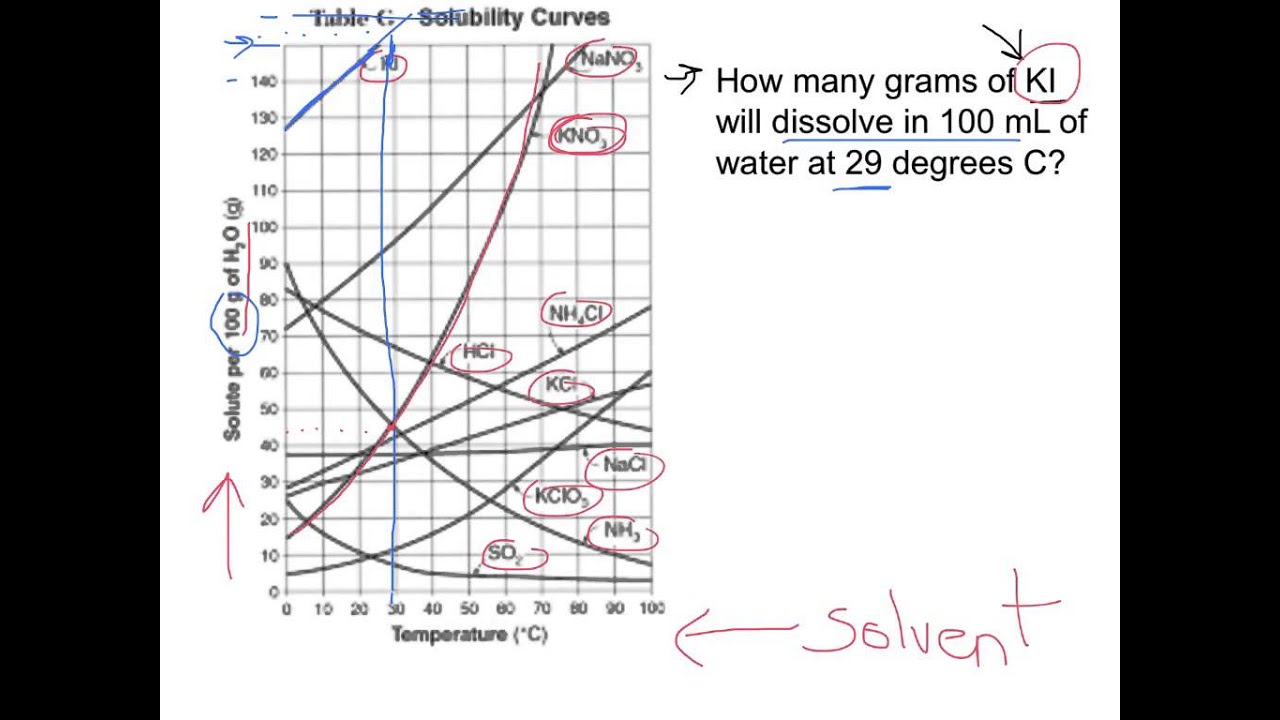
Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. The solubility curve line shows you with a saturated solution. Saturated solution is basically.
Reading solubility curves YouTube
Web how to read the solubility curve? Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. The solubility curve line shows you with a saturated solution. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Solubility curves for more than one substance are.
PPT Solubility curve PowerPoint Presentation, free download ID6497715
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web how to read the solubility curve? The solubility curve line shows you with a saturated solution. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Solubility curves for more than one substance are.
Read Solubility Curve Practice Answers / Hw Solubility Curve Worksheet
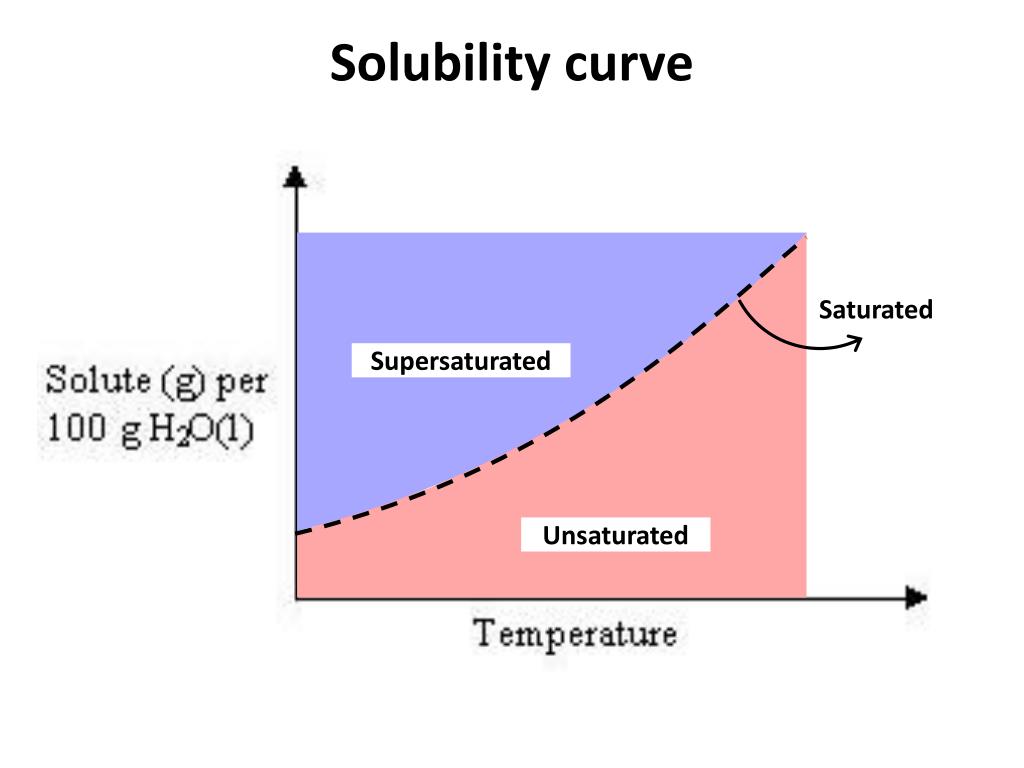
Solubility curves for more than one substance are often drawn on the same. The solubility curve line shows you with a saturated solution. Web how to read the solubility curve? Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find.
Solubility Curve Practice Problems Worksheet 1 Answers Chemistry
Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. A solubility curve is a graph of solubility, measured in g/100 g water, against.
Read Solubility Curve Practice Answers / Solubility Graph Worksheet
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. The solubility curve line shows you with a saturated solution. Web how to read the solubility curve? Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation.
Read Solubility Curve Practice Answers Solubility Curve Worksheet
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web how to read the solubility curve? The solubility curve line shows you with a saturated solution. Saturated solution is basically the one with a full dissolved volume of solute in 100 grams of water. Web since a solubility curve only shows you.
Saturated Solution Is Basically The One With A Full Dissolved Volume Of Solute In 100 Grams Of Water.
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web since a solubility curve only shows you the amount of solute that dissolves in 100g (or 100 ml) of water, there is a handy calculation on how to find the amount needed when dealing with a situation that requires more or less of a solution. The solubility curve line shows you with a saturated solution. Web how to read the solubility curve?