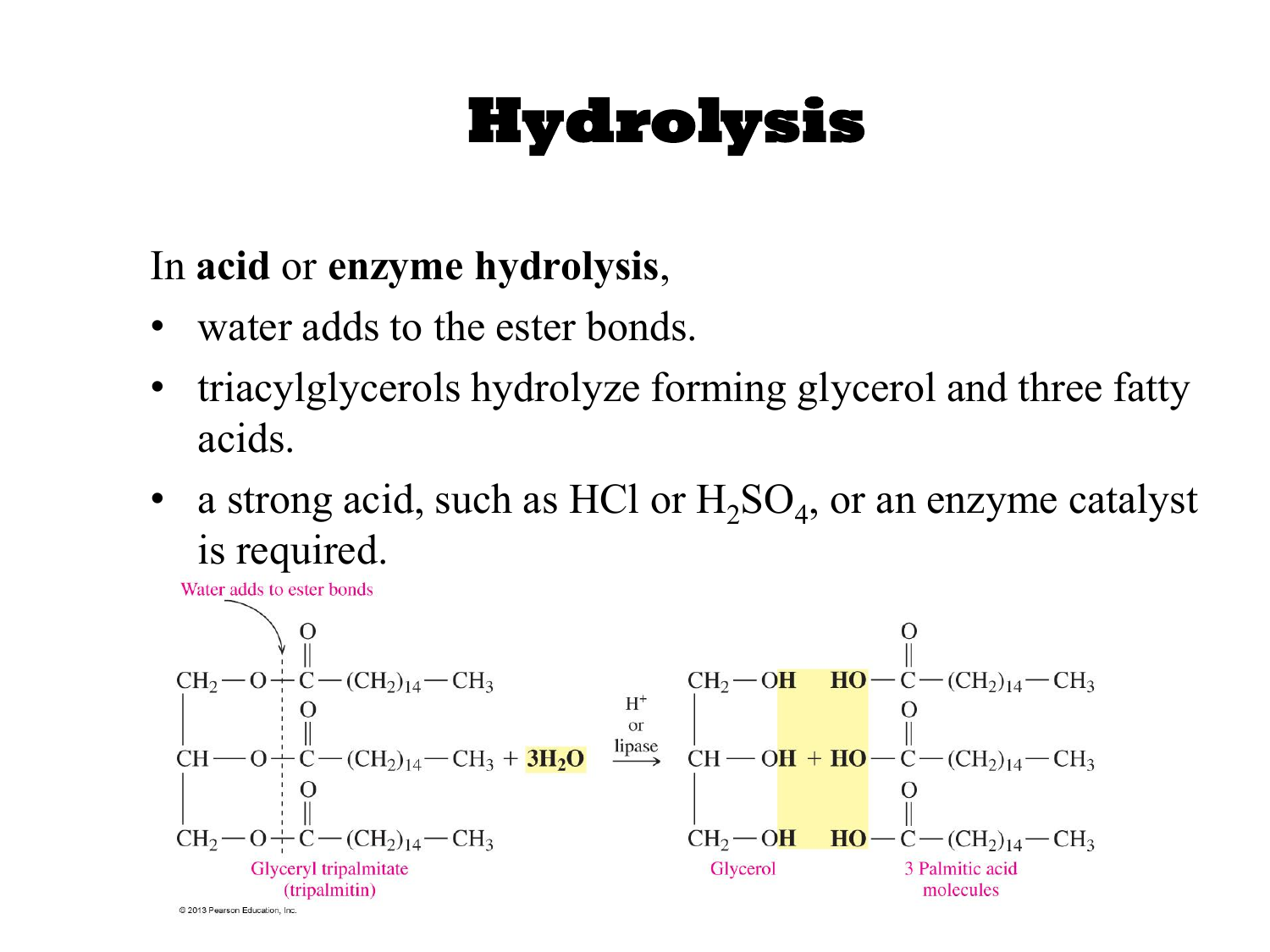
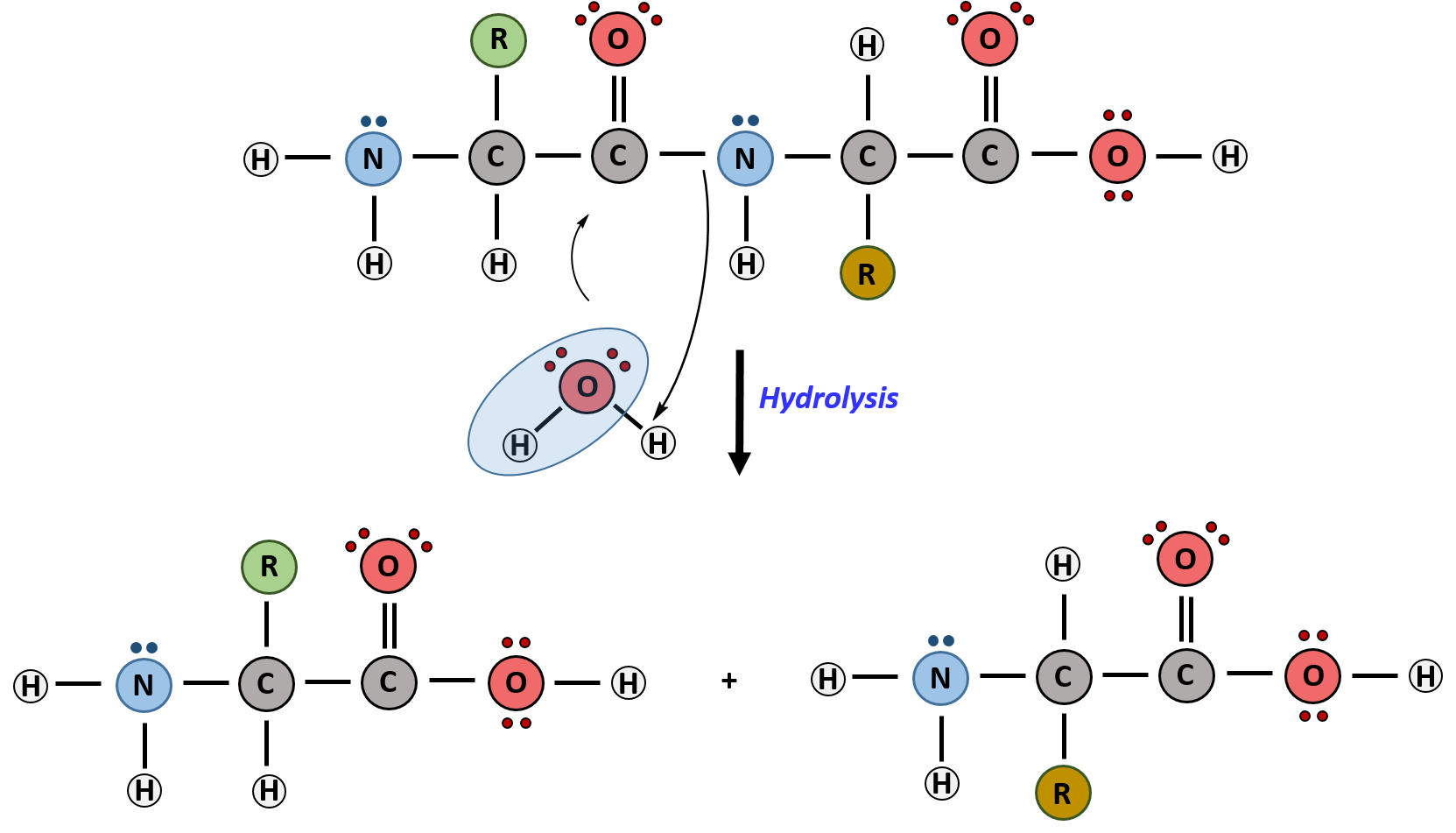
Hydrolysis Drawing
Hydrolysis Drawing - General reaction scheme of a hydrolysis reaction. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Hydrolysis (hydro = water and lysis = break) involves adding water to one large molecule to break it into multiple smaller molecules. Ethanenitrile on getting hydrolysed gives ethanamide in the first step, while. Web what is hydrolysis? | condensation of alcohols | condensation of amines |. A cell can be thought of as a small, bustling town. Draw the structures of the three organic products formed by the complete acid hydrolysis of aspartame. While in the second step, an ammonium salt of a carboxylic acid is formed. Calculate the concentrations of various ions in a salt solution.
Web this acid consists mainly of hydrochloric acid. The addition of sucrose, table sugar, to food and drink has been linked to the increased risk of. Find more free tutorials, videos and readings for the science classroom at ricochetscience.com The reaction is assisted through the use of an acid catalyst. Web explore the fascinating process of atp hydrolysis! The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Hydrolysis (hydro = water and lysis = break) involves adding water to one large molecule to break it into multiple smaller molecules. Web hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. | condensation of alcohols | condensation of amines |. Ethanenitrile on getting hydrolysed gives ethanamide in the first step, while.
| condensation of alcohols | condensation of amines |. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Web hydrolysis of imines to give ketones (or aldehydes) description: A salt is formed between the reaction of an acid and a base. A cell can be thought of as a small, bustling town. Carrier proteins move substances into and out of the cell, motor proteins carry cargoes along microtubule tracks, and metabolic enzymes busily break down and build up macromolecules. Want to join the conversation? Web california institute of technology. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. In this problem you are asked to draw structural formulas for the organic hydrolysis product (s) of the selected compound.
An Explanation of the Process Hydrolysis
| condensation of alcohols | condensation of amines |. Web laguna design / getty images. Web if you use strong acid or a strong base and you heat things up for several hours, you can hydrolyze amides. Ethanenitrile on getting hydrolysed gives ethanamide in the first step, while. The structural formulas of five compounds are drawn below.
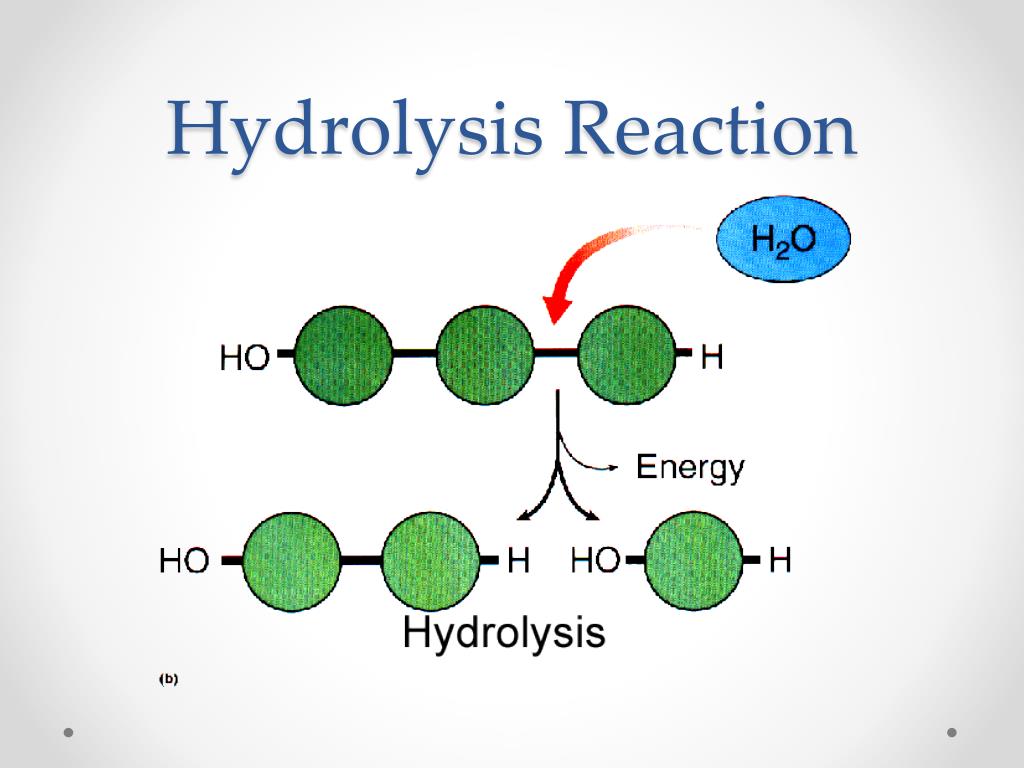
Hydrolysis Reaction Definition, Equation, and Applications
Web california institute of technology. And typically, water is used to. Draw the structures of the three organic products formed by the complete acid hydrolysis of aspartame. Web what is hydrolysis? You may select any one of these by clicking the radio button below the structure.
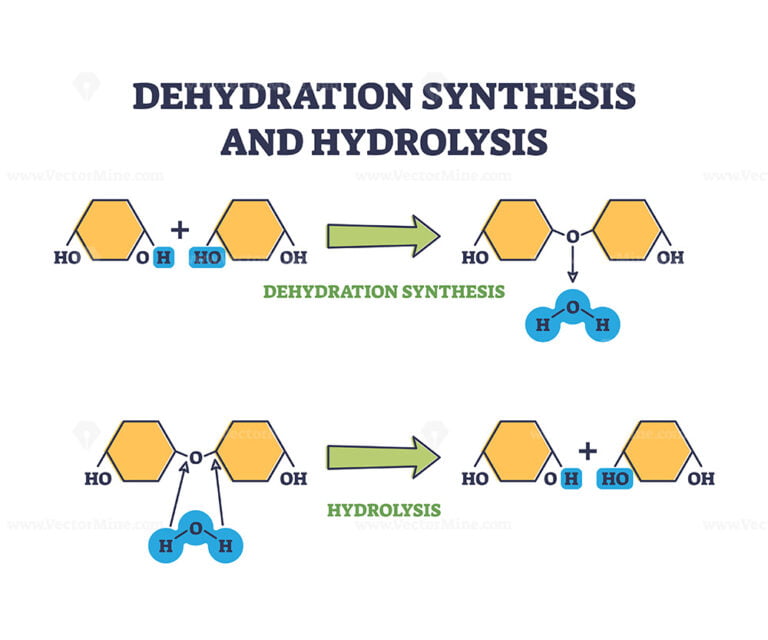
Dehydration synthesis and hydrolysis chemical process stages outline
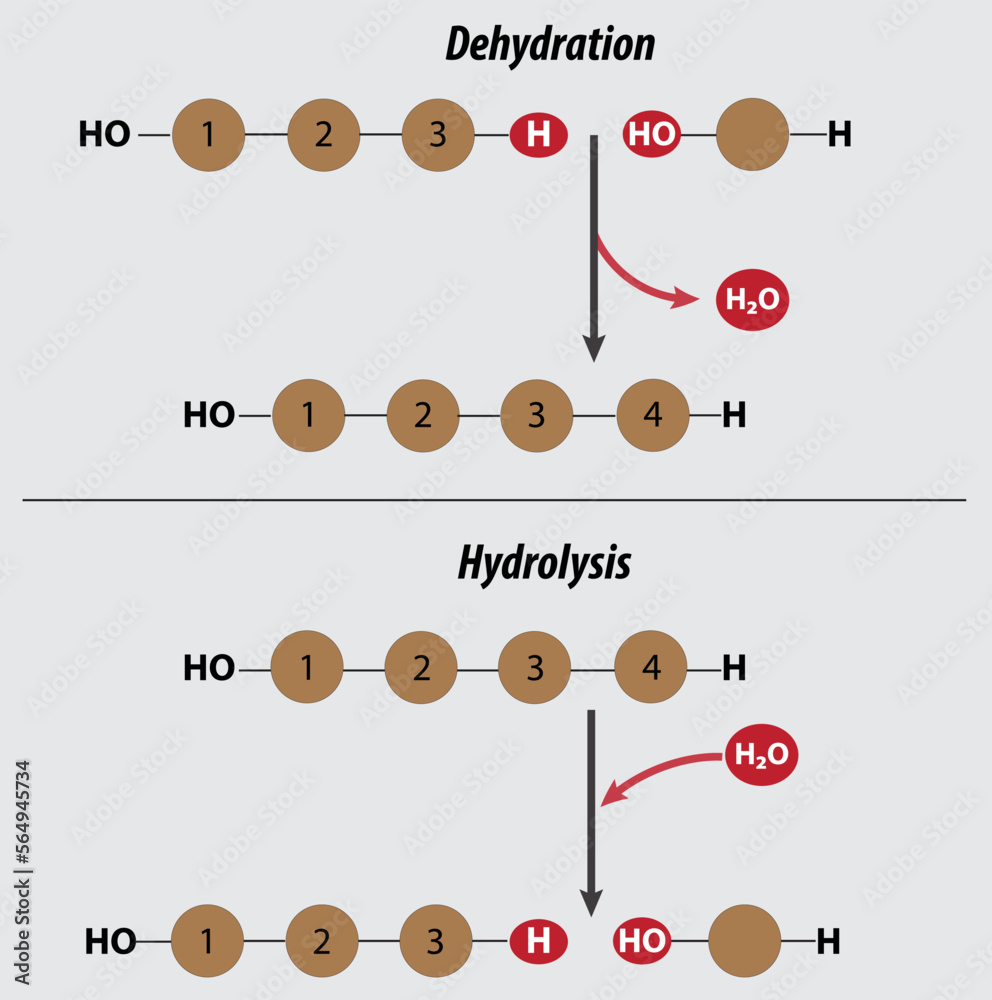
Web in a hydrolysis reaction, a larger molecule forms two (or more) smaller molecules and water is consumed as a reactant. A salt is formed between the reaction of an acid and a base. What is required for hydrolysis? Dive into the detailed mechanism of this vital biological reaction. This occurs through the process of hydrolysis, which uses water to.
Stockvector Dehydration and hydrolysis reactions mnemonic vector
Web explore the fascinating process of atp hydrolysis! Web calculate the ph of a salt solution. Generally, amides can be hydrolyzed in either acidic or basic solution. Hydrolysis (hydro = water and lysis = break) involves adding water to one large molecule to break it into multiple smaller molecules. Want to join the conversation?
PPT Chapter 5 The Molecules of Life PowerPoint Presentation, free
Draw the structures of the three organic products formed by the complete acid hydrolysis of aspartame. Updated on december 08, 2019. It is a chemical mechanism in which, by adding a molecule of water, a molecule is broken into two pieces. During these reactions, the polymer is broken into two components. Hydrolysis (hydro = water and lysis = break) involves.
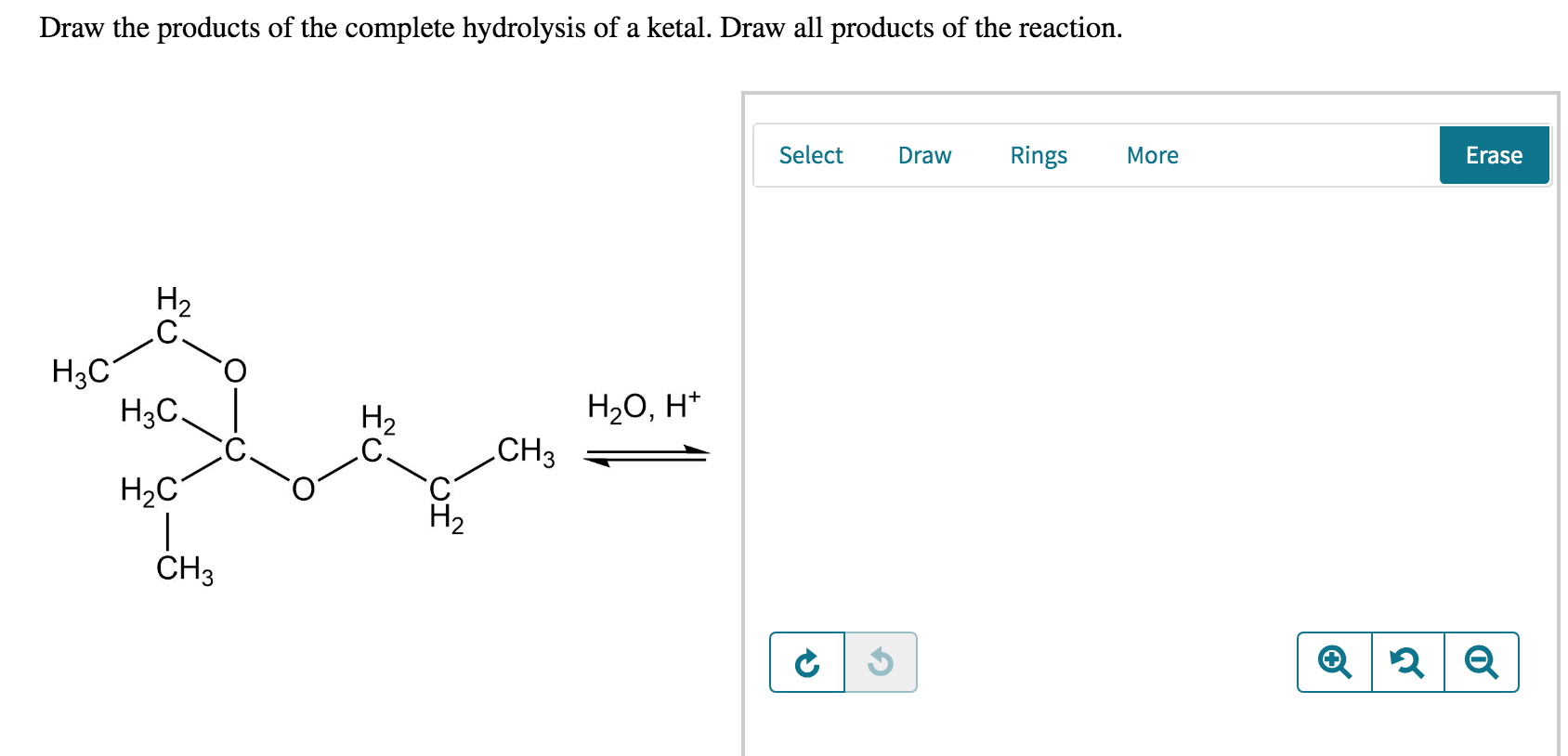
Draw The Products Of Each Hydrolysis Reaction The Expert
And typically, water is used to. Atp structure, atp hydrolysis to adp, and reaction coupling. Web table of content. Web hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. What is required for hydrolysis?
Hydrolysis Diagram Photos
General reaction scheme of a hydrolysis reaction. Identify condensation reactions, and write balanced chemical reactions for the formation of esters and carboxamides. Web hydrolysis of imines to give ketones (or aldehydes) description: Carrier proteins move substances into and out of the cell, motor proteins carry cargoes along microtubule tracks, and metabolic enzymes busily break down and build up macromolecules. Hydrolysis.
Hydrolysis Diagram Photos
The reaction is assisted through the use of an acid catalyst. It is a chemical mechanism in which, by adding a molecule of water, a molecule is broken into two pieces. Want to join the conversation? While in the second step, an ammonium salt of a carboxylic acid is formed. Draw the structures of the three organic products formed by.
Hydrolysis Definition, Examples, & Facts Britannica
Ethanenitrile on getting hydrolysed gives ethanamide in the first step, while. Usually, a neutral salt is formed when a strong acid and a strong base are neutralized in the reaction: Hydrolysis (hydro = water and lysis = break) involves adding water to one large molecule to break it into multiple smaller molecules. Hydrolysis is a type of decomposition reaction where.
Chapter 2 Protein Structure Chemistry
During these reactions, the polymer is broken into two components. This occurs through the process of hydrolysis, which uses water to break the bonds between monosaccharides. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Ethanenitrile on getting hydrolysed gives ethanamide in the first step, while. | hydrolysis of esters | hydrolysis.
Thus, If A Compound Is Represented By The Formula Ab In Which A And B Are Atoms Or Groups And Water Is Represented By The Formula Hoh, The Hydrolysis Reaction May Be Represented By The Reversible Chemical Equation Ab + Hoh.
Web table of content. The entire molecule changes its structure as new bonds are formed. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water, this is an example of a “condensation reaction”. Web hydrolysis ( / haɪˈdrɒlɪsɪs /;
It Is A Chemical Mechanism In Which, By Adding A Molecule Of Water, A Molecule Is Broken Into Two Pieces.
Hydrolysis is a type of decomposition reaction where one of the reactants is water; Ethanenitrile on getting hydrolysed gives ethanamide in the first step, while. Web hydrolysis, also known as hydrolysis reaction, is a type of decomposition reaction in which a molecule is broken down into components by adding water. Hydrolysis (hydro = water and lysis = break) involves adding water to one large molecule to break it into multiple smaller molecules.
Generally, Amides Can Be Hydrolyzed In Either Acidic Or Basic Solution.
Dive into the detailed mechanism of this vital biological reaction. Calculate the concentrations of various ions in a salt solution. Atp structure, atp hydrolysis to adp, and reaction coupling. Usually, a neutral salt is formed when a strong acid and a strong base are neutralized in the reaction:
In This Problem You Are Asked To Draw Structural Formulas For The Organic Hydrolysis Product (S) Of The Selected Compound.
If we take a look at this amide right here, we can break this bond using acid and heat and form a carboxylic acid. While in the second step, an ammonium salt of a carboxylic acid is formed. Hydrolysis is a chemical reaction in which a compound reacts with water which ultimately leads breakage of bonds within that compound. | hydrolysis of esters | hydrolysis of amides |.
:max_bytes(150000):strip_icc()/what-is-hydrolysis-375589-v2-5bdb527846e0fb002d706279.png)