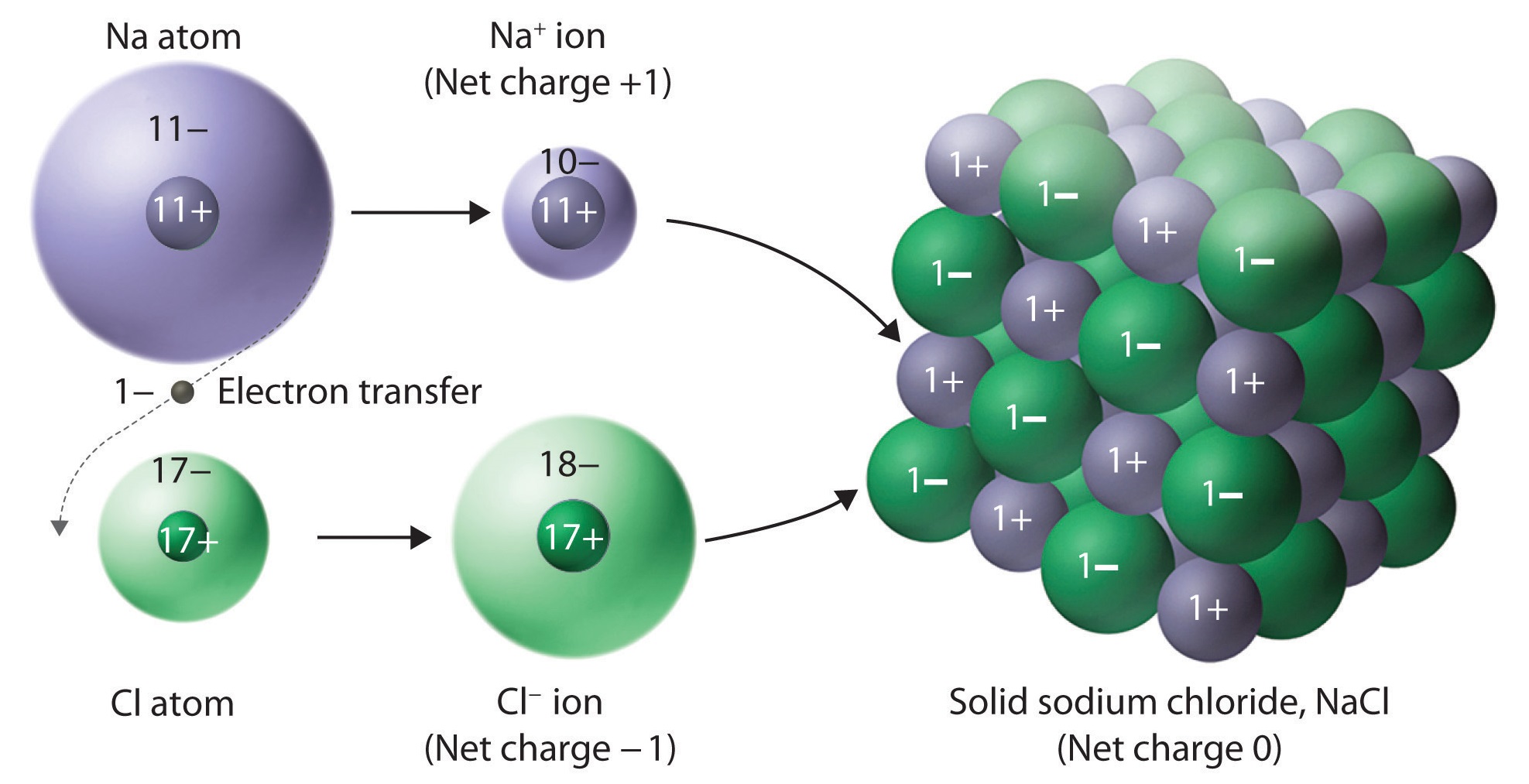
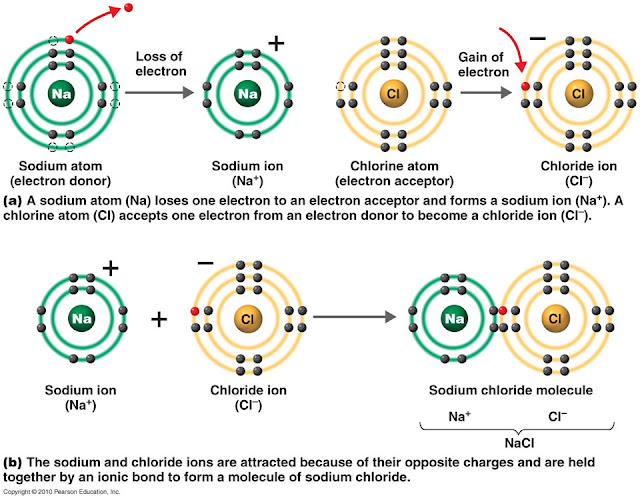
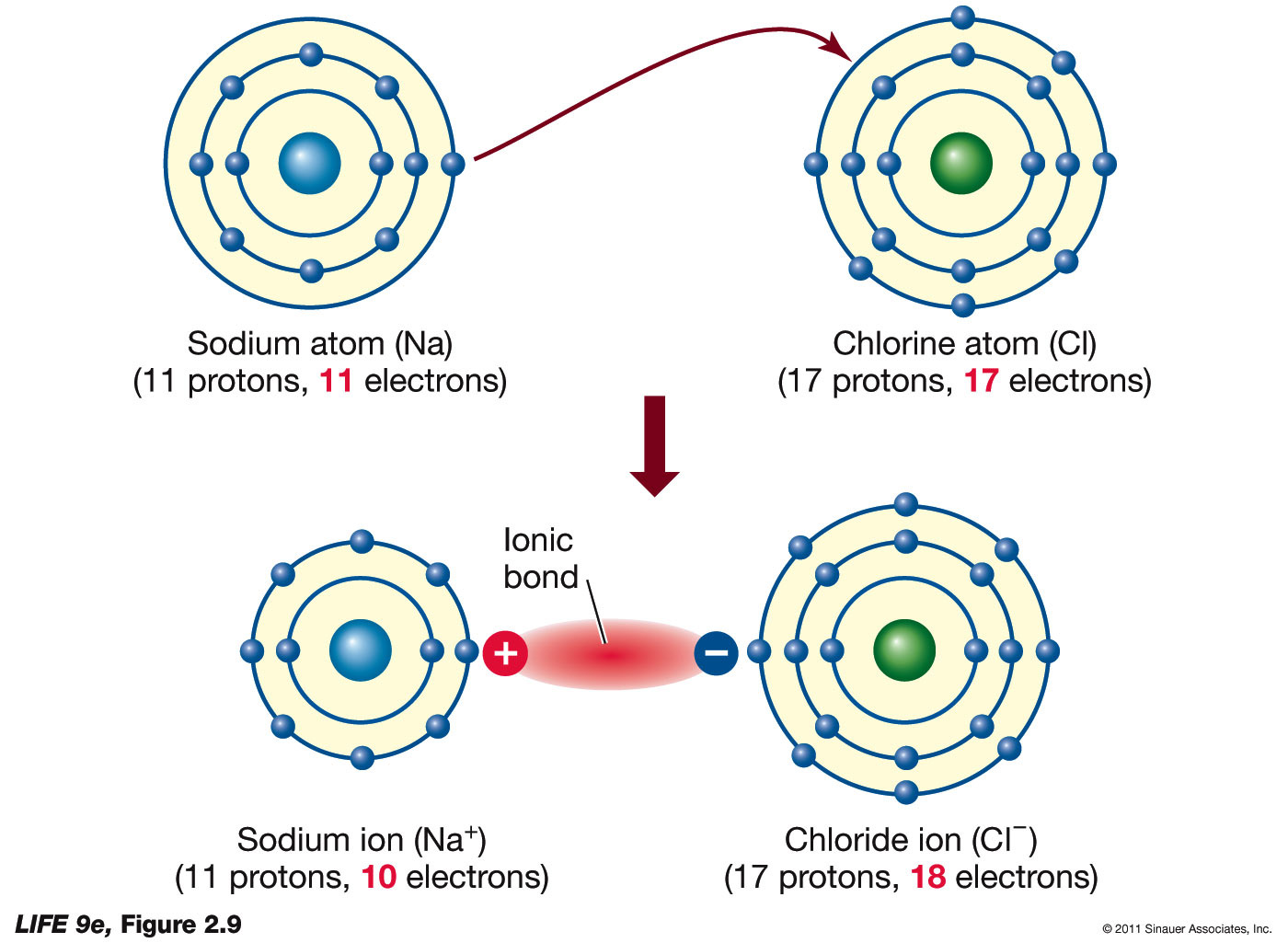
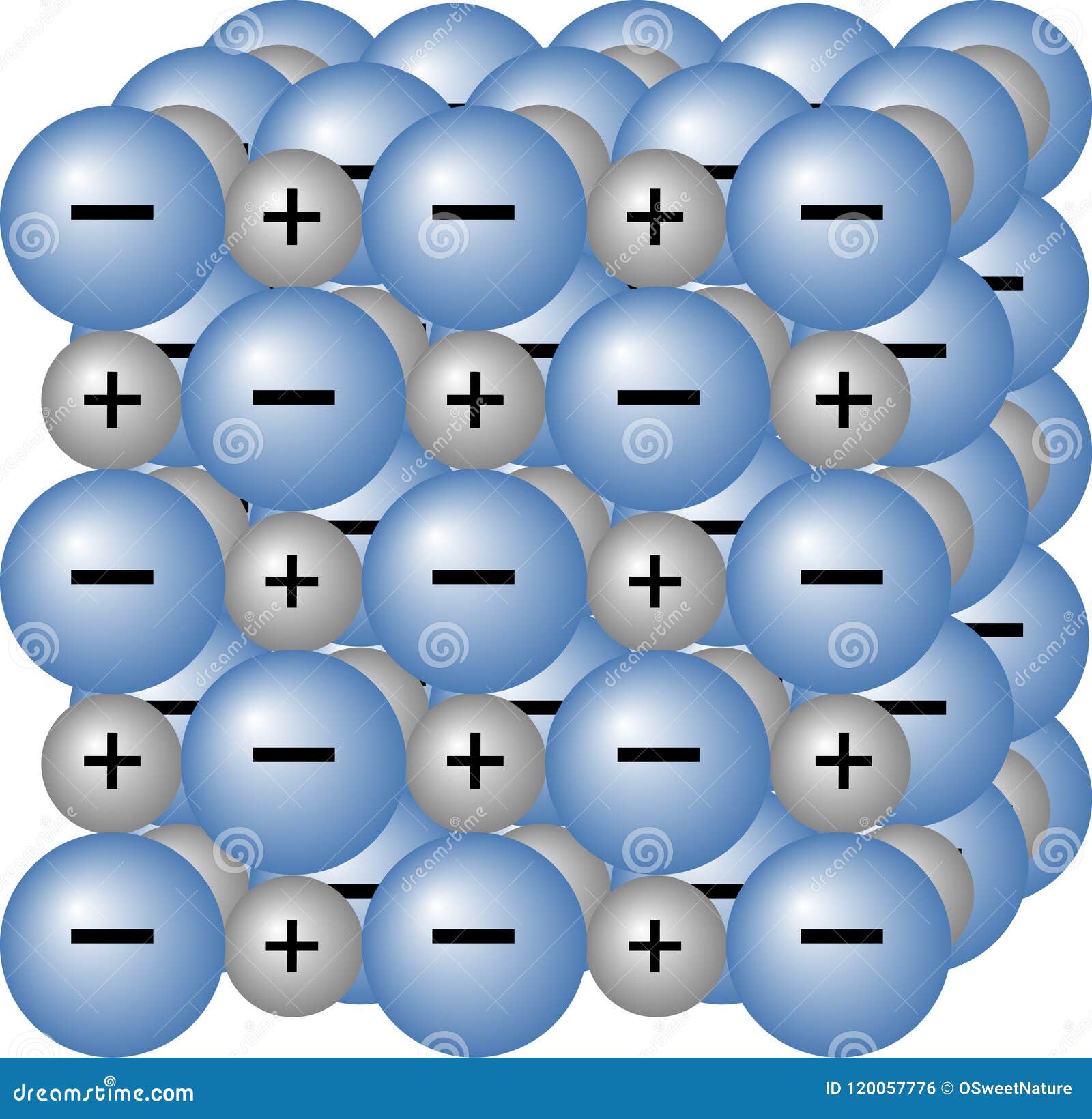
Ionic Compound Drawing
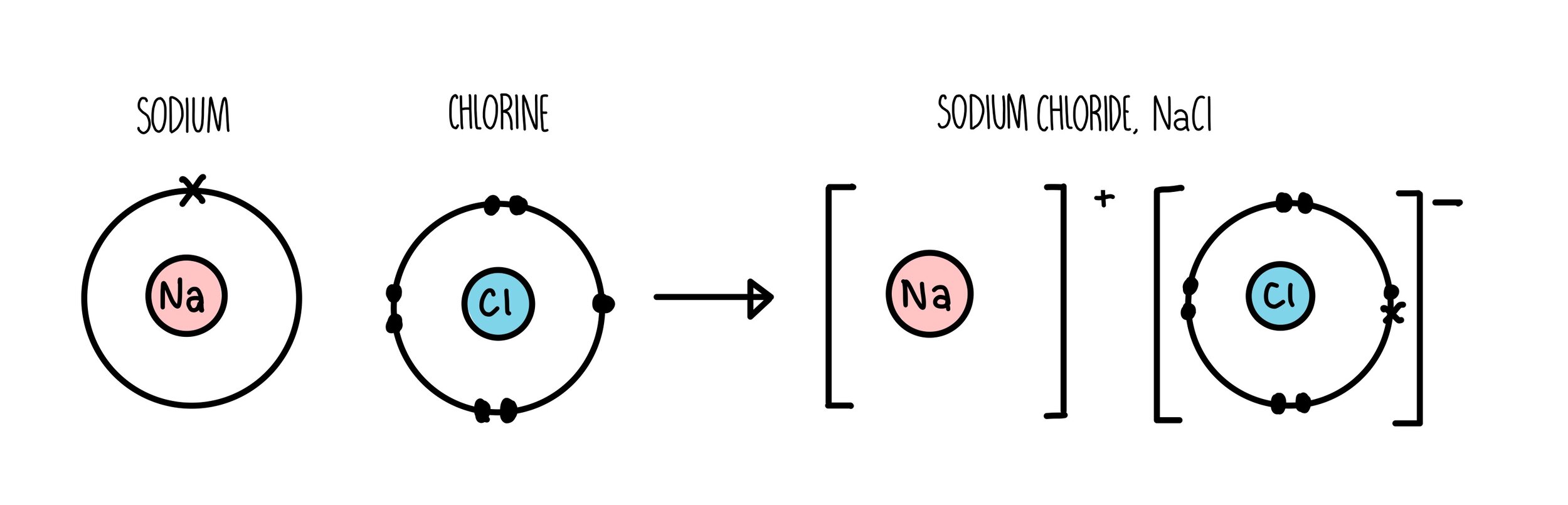
Ionic Compound Drawing - The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Polyatomic ions are ions comprised of more than one atom. Covalent compounds usually form from two or more nonmetals. The basics of gob chemistry textmap. A good example is the ammonium ion made up of one nitrogen atom and four hydrogen atoms. Web we recommend you use a larger device to draw your structure. Web this chemistry video explains how to draw the lewis structures of ionic compounds. Web to draw a dot and cross diagram of an ionic compound, draw dot and cross diagrams of the ions involved, in the correct ratio. Web for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge.
Web for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Group one of the periodic table contains l i superscript plus sign in period 2, n a superscript plus sign in period 3, k superscript plus sign. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Draw the lewis symbol for each ion, representing the valence electrons as dots or. With that, the formula of sodium. Identify the elements involved in the compound and determine their respective valence electrons. Smiles smarts inchi mdl molfile isis sketch isis tgf chemdraw cdx chemdraw xml cml mrv sybyl sln jme smd png image pict image gif image wmf image svg image eps image mif image swf image pdf image.
Then you would draw them separately and put a bracket around each of them. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Group one of the periodic table contains l i superscript plus sign in period 2, n a superscript plus sign in period 3, k superscript plus sign. In a precipitation reaction, a solid product is formed from two electrolyte solutions. Much of the study of chemistry, however, involves looking at what happens. Web shows how to draw lewis dot structures for ionic compounds. Developed with the support of minnesota state colleges and universities. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. The tendency to form species that have eight electrons in the valence shell. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell.
What Is An Ionic Compound? Formula and Defination
Much of the study of chemistry, however, involves looking at what happens. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. A lewis diagram shows how the valence electrons are distributed around the atoms in a.
Ionic Properties
Developed with the support of minnesota state colleges and universities. The skeletal structure of ethanethiol is shown below. Draw the lewis diagram for the molecules, hydrogen chloride, brcl, and hydrogen cyanide (hcn). The tendency to form species that have eight electrons in the valence shell. Polyatomic ions are ions comprised of more than one atom.
Ionic Solids Chemistry LibreTexts
Web draw a cartoon of an ionic compound that partially dissolves in water. Developed with the support of minnesota state colleges and universities. Web to draw a dot and cross diagram of an ionic compound, draw dot and cross diagrams of the ions involved, in the correct ratio. The structural formula editor is surround by three toolbars which contain the.
What are Ionic Compounds and how they are formed?
The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. These are homework exercises to accompany chapter 3 of the ball et al. Identify the elements involved in the compound and determine their respective.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Then you would draw them separately and put a bracket around each of them. Try rotating the device so that it is in a landscape position. Web for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. Smiles smarts inchi mdl molfile isis sketch isis tgf chemdraw.
Lewis Dot Structure Ionic Compounds
Web the empirical formula for any ionic compound is the smallest unit which repeats in an ionic solid. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Examples include nacl, mgf2, k2o,.
Examples of Ionic Bonds and Compounds
Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web the empirical formula for any ionic compound is the smallest unit which repeats in an ionic solid. Try rotating the device so that it is in a landscape position. Use dots for one ion's electrons and crosses.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Web this chemistry video explains how to draw the lewis structures of ionic compounds. Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent.
Diagram of an Ionic Compound Stock Illustration Illustration of
Swap the crosses for dots in one of your diagrams. Web an introduction to drawing lewis structures of ionic compounds in masteringchemistry. Smiles smarts inchi mdl molfile isis sketch isis tgf chemdraw cdx chemdraw xml cml mrv sybyl sln jme smd png image pict image gif image wmf image svg image eps image mif image swf image pdf image. Shared.
Ionic Bonding — the science hive
Examples include nacl, mgf2, k2o, and al2o3.how to draw lewis structures:. Web exercise 3.3.1 3.3. Web the empirical formula for any ionic compound is the smallest unit which repeats in an ionic solid. We can draw what we could call particulate models. Smiles smarts inchi mdl molfile isis sketch isis tgf chemdraw cdx chemdraw xml cml mrv sybyl sln jme.
Once You’ve Drawn A Molecule, You Can Click The 2D To 3D Button To Convert The Molecule Into A 3D Model Which Is Then.
Group one of the periodic table contains l i superscript plus sign in period 2, n a superscript plus sign in period 3, k superscript plus sign. Determine the charge of each ion based on the element's position in the periodic table. With that, the formula of sodium. The tendency to form species that have eight electrons in the valence shell.
Magnesium Oxide Dot & Cross Diagram.
Since sodium is in group i of the periodic table, it forms ions with charge of +1. Web draw the outer shell of each atom. Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Covalent compounds usually form from two or more nonmetals.
Web The Strength Of Ionic Bonding Depends On The Magnitude Of The Charges And The Sizes Of The Ions.
A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Polyatomic ions are ions comprised of more than one atom. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Same skeleton (including h) same skeleton (excluding h)
Web The Empirical Formula For Any Ionic Compound Is The Smallest Unit Which Repeats In An Ionic Solid.
Which of the following correctly completes the lewis. Web we recommend you use a larger device to draw your structure. Draw the lewis symbol for each ion, representing the valence electrons as dots or. The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks.