Most Oxidized Form Of Carbon
Most Oxidized Form Of Carbon - Web through their combined efforts, the carbon present in various organic compounds is progressively transformed to its most oxidized form, that of carbon. What are the oxidation numbers? However, looking at the structure of carbonic acid or carbonate, it seems that. Its commonly said that co2 is the most oxidized form of carbon. Explore more crossword clues and. Web most oxidized state of carbon? They have 4 and 6 valence electrons respectively. Numerous additional unstable or metastable oxides, on the other. Tetrahydrofolate is involved in single carbon transfer bound to positions n5, n10, or both. You'll get a detailed solution.
Web the formation of carbon oxides is due to electronic configurations of carbon and oxygen. Web definition chemoautotrophs are organisms that obtain their energy from a chemical reaction (chemotrophs) but their source of carbon is the most oxidized form of carbon, carbon. The oxidation state of an atom is a measure of the degree of oxidation of an atom. Web which of the following molecules contains the most oxidized form of carbon? Tetrahydrofolate is involved in single carbon transfer bound to positions n5, n10, or both. Its commonly said that co2 is the most oxidized form of carbon. Web carbon is a chemical element having the symbol capital c. However, looking at the structure of carbonic acid or carbonate, it seems that. What are structures corresponding to the most oxidized and most reduced forms of carbon. What are the oxidation numbers?
Web i saw an interesting spectrum in my biology class the other day. Web carbon monoxide (co) and carbon dioxide (co2) are the two most often encountered carbon oxides (co2). Its commonly said that co2 is the most oxidized form of carbon. Web which of the following molecules contains the most oxidized form of carbon? Web co 2 is a moderately stable gas with very low reactivity, since it is the most oxidized form of carbon. They have 4 and 6 valence electrons respectively. Web the most reduced form of carbon is ch4, the most oxidized is co2. What are structures corresponding to the most oxidized and most reduced forms of carbon. Web the most highly oxidized form of carbon is: However, looking at the structure of carbonic acid or carbonate, it seems that.
Unexpected deepEarth oxidized iron surprises geologists
Web which of the following molecules contains the most oxidized form of carbon? What are the oxidation numbers? Carbon is one of the most important elements in living organisms and industries as it is used to form fuel, gas, and. Web carbon is a chemical element having the symbol capital c. Web the most highly oxidized form of carbon is:
Calculating the oxidation state of a carbon Master Organic Chemistry
Carbon is one of the most important elements in living organisms and industries as it is used to form fuel, gas, and. You'll get a detailed solution. Web i saw an interesting spectrum in my biology class the other day. What are the oxidation numbers? Web which of the following molecules contains the most oxidized form of carbon?
Oxidized minerals form the multicolored Artist's Palette along Artists
A) acetaldehyde b) ethanol c) acetic acid d) ethylene e) carbon dioxide |. Web which of the following molecules contains the most oxidized form of carbon? Web for the word puzzle clue of are most forms of cycling carbon reduced or oxidized, the sporcle puzzle library found the following results. Web carbon is a chemical element having the symbol capital.
Solved Rank The Following Compounds On The Basis Of The O...
Web most oxidized state of carbon? Its commonly said that co2 is the most oxidized form of carbon. Web through their combined efforts, the carbon present in various organic compounds is progressively transformed to its most oxidized form, that of carbon. Explore more crossword clues and. Web view the full answer.
Solved 1.Using The Compounds Below A. Arrange The Compou...
Web for the word puzzle clue of are most forms of cycling carbon reduced or oxidized, the sporcle puzzle library found the following results. What are structures corresponding to the most oxidized and most reduced forms of carbon. It is defined as being the charge that an atom would have if all. Numerous additional unstable or metastable oxides, on the.
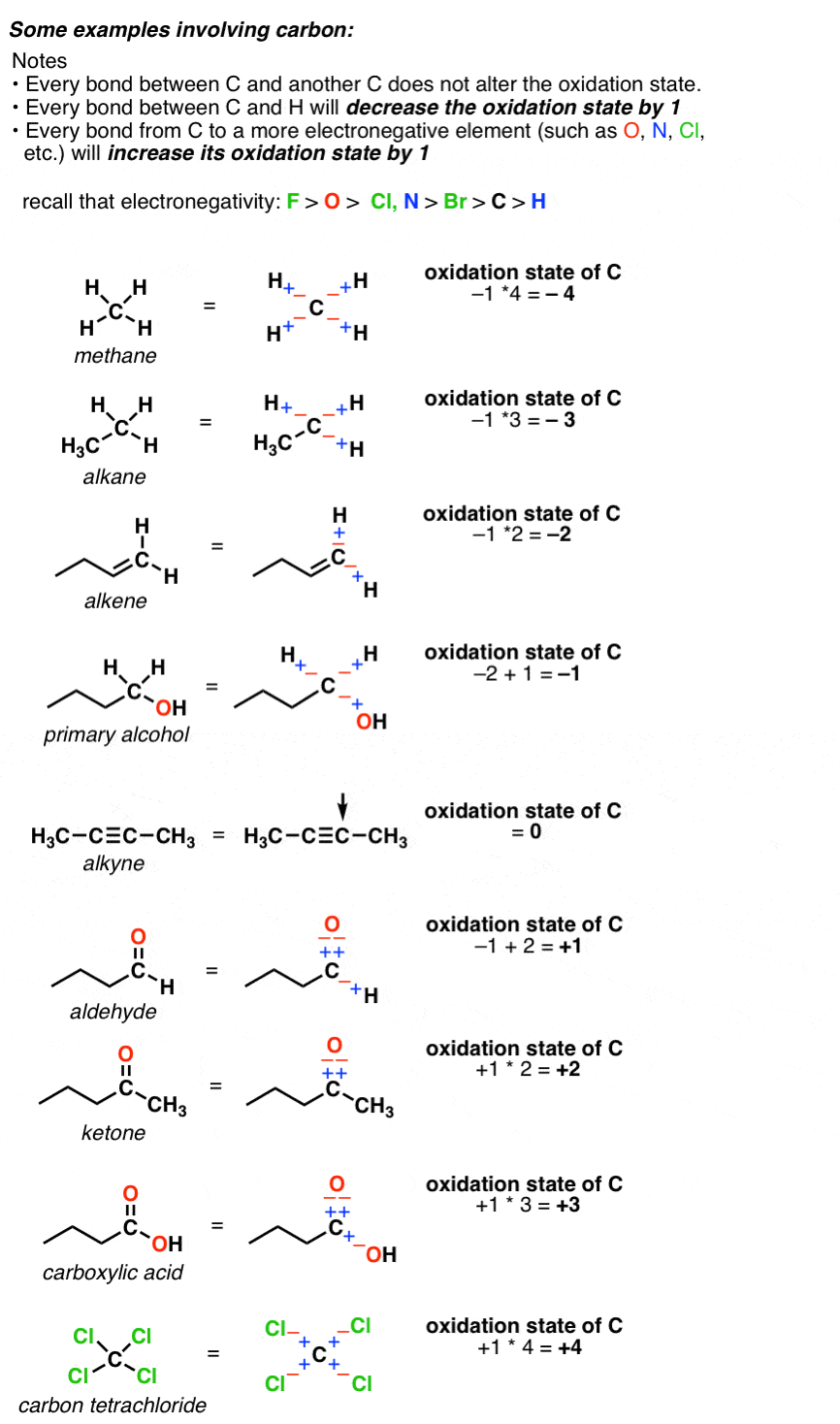
Solved Rank the following from least to most oxidized 1
Web the most highly oxidized form of carbon is: It is defined as being the charge that an atom would have if all. Its commonly said that co2 is the most oxidized form of carbon. Web carbon is a chemical element having the symbol capital c. Numerous additional unstable or metastable oxides, on the other.
Energy from Fossil Fuels
Web through their combined efforts, the carbon present in various organic compounds is progressively transformed to its most oxidized form, that of carbon. Web which of the following molecules contains the most oxidized form of carbon? Web most oxidized state of carbon? The oxidation state of an atom is a measure of the degree of oxidation of an atom. Numerous.
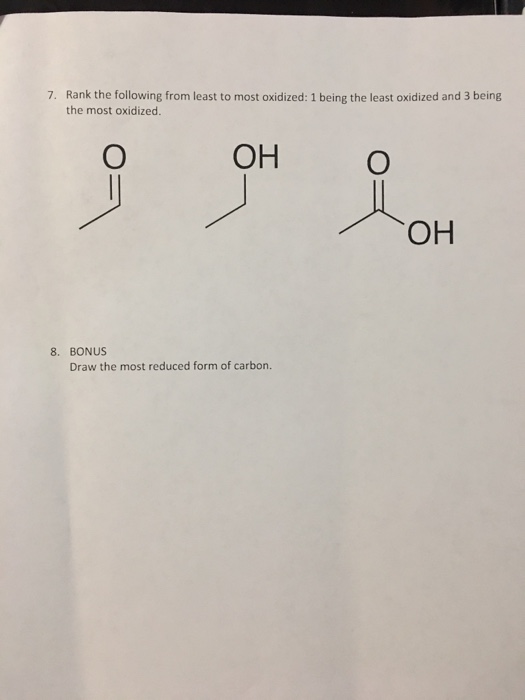
Solved 2. Rank the following carbon compounds from most
It is defined as being the charge that an atom would have if all. Web definition chemoautotrophs are organisms that obtain their energy from a chemical reaction (chemotrophs) but their source of carbon is the most oxidized form of carbon, carbon. Explore more crossword clues and. Web carbon monoxide (co) and carbon dioxide (co2) are the two most often encountered.
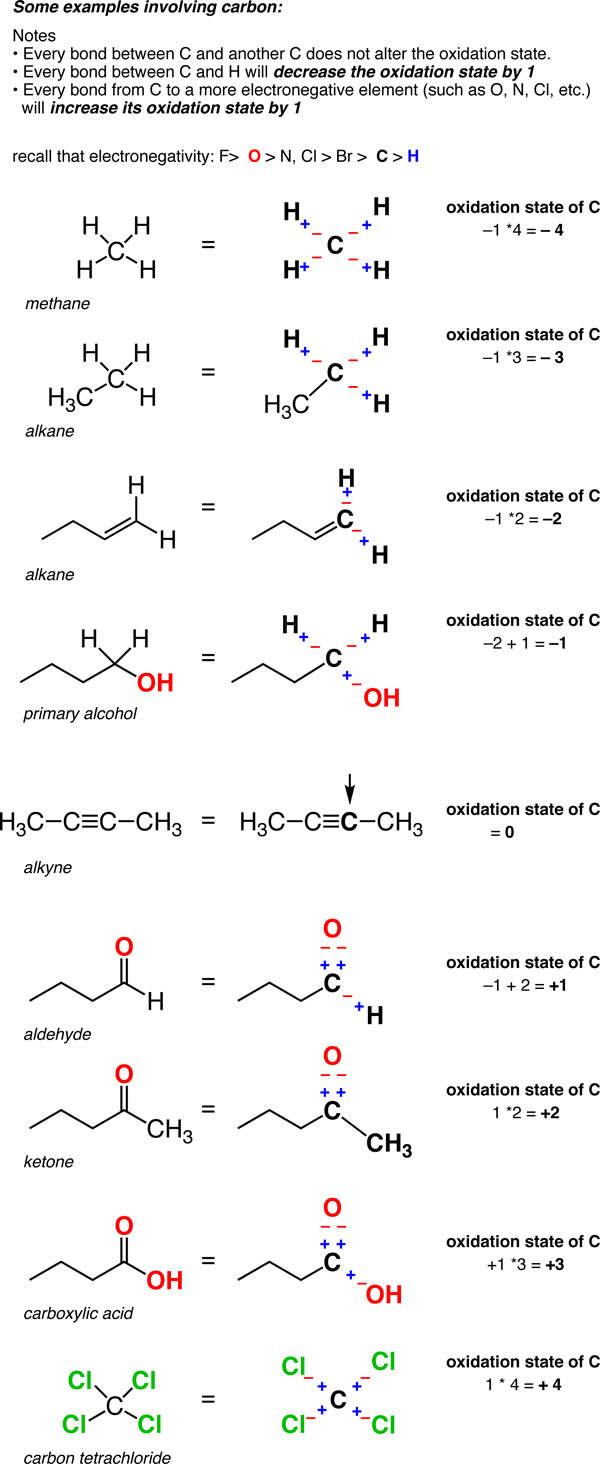
19.1. Definition of oxidation state for carbon Organic Chemistry II
What are the oxidation numbers? Carbon in protein hydrocarbons carbohydrate carbon dioxide charcoal this problem has been solved! Web definition chemoautotrophs are organisms that obtain their energy from a chemical reaction (chemotrophs) but their source of carbon is the most oxidized form of carbon, carbon. However, looking at the structure of carbonic acid or carbonate, it seems that. Web view.
Solved Arrange the compounds from least oxidized to most
They have 4 and 6 valence electrons respectively. Its commonly said that co2 is the most oxidized form of carbon. It is defined as being the charge that an atom would have if all. You'll get a detailed solution. Web carbon is a chemical element having the symbol capital c.
Explore More Crossword Clues And.
Web which of the following molecules contains the most oxidized form of carbon? However, looking at the structure of carbonic acid or carbonate, it seems that. What are structures corresponding to the most oxidized and most reduced forms of carbon. Tetrahydrofolate is involved in single carbon transfer bound to positions n5, n10, or both.
You'll Get A Detailed Solution.
Web carbon monoxide (co) and carbon dioxide (co2) are the two most often encountered carbon oxides (co2). The oxidation state of an atom is a measure of the degree of oxidation of an atom. Numerous additional unstable or metastable oxides, on the other. Web view the full answer.
Its Commonly Said That Co2 Is The Most Oxidized Form Of Carbon.
Web the most reduced form of carbon is ch4, the most oxidized is co2. Web most oxidized state of carbon? Web definition chemoautotrophs are organisms that obtain their energy from a chemical reaction (chemotrophs) but their source of carbon is the most oxidized form of carbon, carbon. It is defined as being the charge that an atom would have if all.
Web Carbon Is A Chemical Element Having The Symbol Capital C.
Carbon is one of the most important elements in living organisms and industries as it is used to form fuel, gas, and. Web i saw an interesting spectrum in my biology class the other day. They have 4 and 6 valence electrons respectively. What are the oxidation numbers?