Quiz 5 Chapter Review Chemistry
Quiz 5 Chapter Review Chemistry - The lewis dot symbol consists of the symbol for the element surrounded by dot(s). Unit 5 chemical reactions and stoichiometry. The symbols for the atoms. What is the total number of energy levels used by an atom of aluminum (al, atomic #13) in the ground state?. Web use these quizzes to test yourself on how well you know chemistry. The formula of a compound indicates all of the following except: Click the card to flip 👆. Web learn created by anna1735 terms in this set (97) 1. Special project* 8 molecular structure 19. Chemical reactions, rates, and equilibrium 20.
Unit 2 more about atoms. Web make sure your answer is the same as the number of significant figures as the starting value. Preview this quiz on quizizz. 1.8 (39 reviews) the arrangement of the atoms in the molecule. Atom forms a cation when it. Web use these quizzes to test yourself on how well you know chemistry. The p block metals are generaly harder and more dense then the s block metals but softer and less. Mendeleev organized the elements by. All responses must be submitted through mastering chemistry. Unit 3 more about molecular composition.
The p block metals are generaly harder and more dense then the s block metals but softer and less. Web use these quizzes to test yourself on how well you know chemistry. The number of valence electrons for 1s 2 2s 2 2p 4 is: Web make sure your answer is the same as the number of significant figures as the starting value. Web chapter 5 chemistry quiz. The lewis dot symbol consists of the symbol for the element surrounded by dot(s). A chemical bond that is made by sharing electrons. The idea of arranging the elements in the periodic table according to their chemical and physical properties is attributed to a. Preview this quiz on quizizz. Chapter review the nuclear product of radioactive decay is called the daughter nuclide.
Quiz 5 Chapter 21 Multiple Choice Identify the choice that best
Click the card to flip 👆. Web use these quizzes to test yourself on how well you know chemistry. The study of the composition of matter and the changes that matter undergoes. Ion combines easily with anions. Chapter review flashcards 2 days ago quizlet.com.
(PDF) Quiz 5 Chapter 2 SOLUTIONS Ani H Academia.edu
Unit 1 atoms, compounds, and ions. Chemical reactions, rates, and equilibrium 20. Ion combines easily with anions. Preview this quiz on quizizz. Web unit 5 test review quiz for 10th grade students.
Chemistry Quiz Appstore for Android
Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium. The lewis dot symbol consists of the symbol for the element surrounded by dot(s). Chapter review learn with flashcards, games, and more — for free. Web play this game to review atoms & molecules. Atom forms a cation when it.
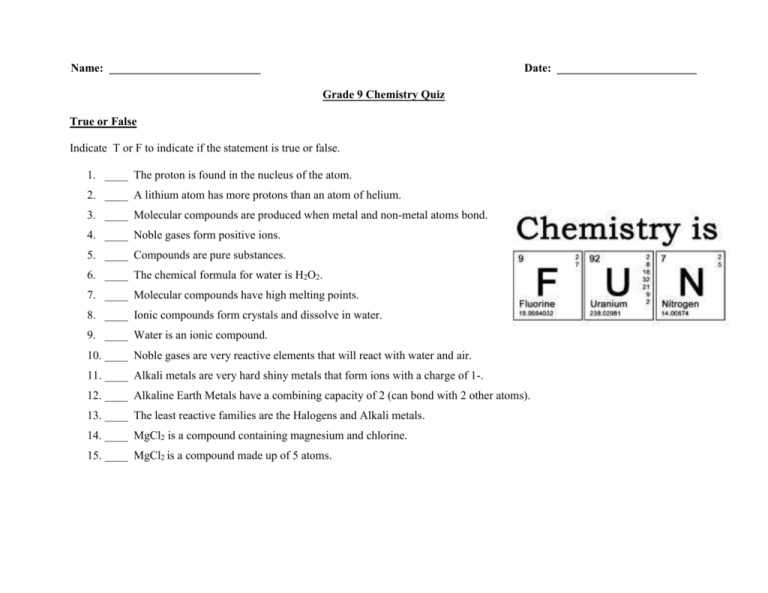
Grade 9 Chemistry Quiz
Core electrons valence electrons atomic number atomic mass. The study of the composition of matter and the changes that matter undergoes. What is the total number of energy levels used by an atom of aluminum (al, atomic #13) in the ground state?. Chemical reactions, rates, and equilibrium 20. The formula of a compound indicates all of the following except:
24+ Quiz 5 Chapter Review NaadeinIwan
Web chapter 5 chemistry quiz. Unit 5 chemical reactions and stoichiometry. Nature's building blocks quiz 5: Graphite and diamond are both composed of __. Click the card to flip 👆.
Test Review Answers
The lewis dot symbol consists of the symbol for the element surrounded by dot(s). All responses must be submitted through mastering chemistry. 1.8 (39 reviews) the arrangement of the atoms in the molecule. Web chemistry library 20 units · 54 skills. A chemical bond that is made by sharing electrons.
Chapter 5 Chapter Review Question Answers
Measurement to gases and moles 16. Web learn created by anna1735 terms in this set (97) 1. Click the card to flip 👆. What is the total number of energy levels used by an atom of aluminum (al, atomic #13) in the ground state?. Chapter review learn with flashcards, games, and more — for free.
24+ Quiz 5 Chapter Review NaadeinIwan
What is the total number of energy levels used by an atom of aluminum (al, atomic #13) in the ground state?. Unit 2 more about atoms. Chemical reactions, rates, and equilibrium 20. Graphite and diamond are both composed of __. A large piece of salt has the same unit cell as fine grains of the same salt.
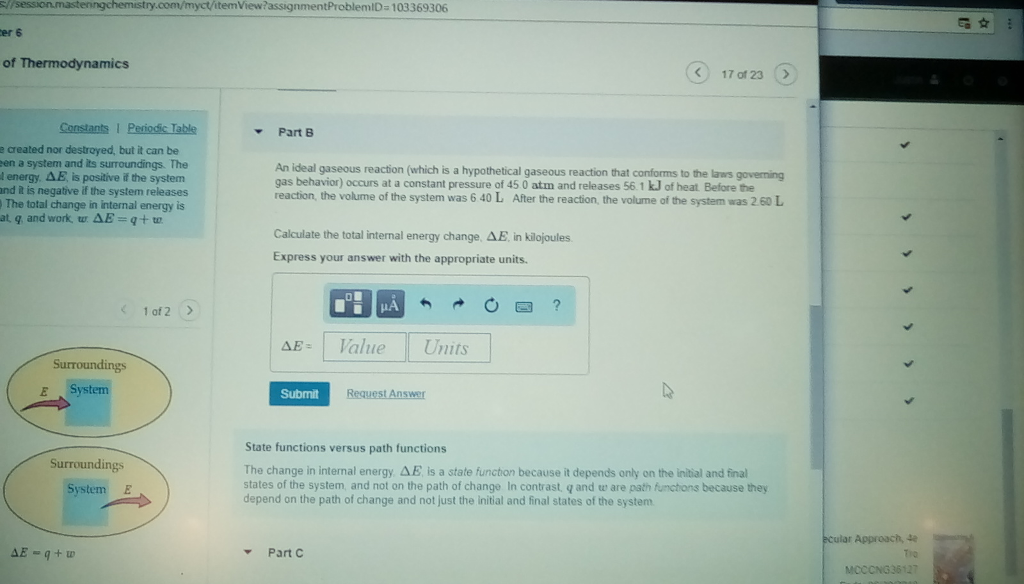
Solved Mastering Chemistry Quiz 5 Chapter 6 Google Ch...
Ion combines easily with anions. Web chemistry chapter 5 review. The number of valence electrons for 1s 2 2s 2 2p 4 is: Find other quizzes for chemistry and more on quizizz for free! Unit 1 atoms, compounds, and ions.
Chemistry Quiz New Gen Entertainments
Graphite and diamond are both composed of __. Preview this quiz on quizizz. Ion combines easily with anions. Web make sure your answer is the same as the number of significant figures as the starting value. The p block metals are generaly harder and more dense then the s block metals but softer and less.
How Do The Properties Of The P Block Metals Compare With Those Of The Metals In The S And D Blocks.
Web unit 5 test review quiz for 10th grade students. All responses must be submitted through mastering chemistry. 1.8 (39 reviews) the arrangement of the atoms in the molecule. What does the dot or dots represent?
Chemical Reactions, Rates, And Equilibrium 20.
The symbols for the atoms. Unit 2 more about atoms. Web play this game to review atoms & molecules. Chapter review the nuclear product of radioactive decay is called the daughter nuclide.
Graphite And Diamond Are Both Composed Of __.
Atom forms a cation when it. We’ve provided hundreds of chemistry questions for you to prepare for your next chemistry quiz or test. The formula of a compound indicates all of the following except: Chapter review learn with flashcards, games, and more — for free.
Unit 1 Atoms, Compounds, And Ions.
Chapter review flashcards 2 days ago quizlet.com. Preview this quiz on quizizz. Web chemistry unit 3 quiz 5. Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium.