What Ion Does Oxygen Form
What Ion Does Oxygen Form - It gains two electrons from one or two other atoms in reactions, forming. This form is called oxyhemoglobin; It has 3 each of protons, neutrons and electrons, and represents that element lithium (li). Web an active form of oxygen, presumably monatomic, has been produced by passing oxygen gas saturated with water vapour through a discharge tube. Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer of electrons. Web answer (1 of 6): Web oxygen forms heteropoly acids and polyoxometalate ions with tungsten, molybdenum and some other transition metals, such as phosphotungstic acid (h 3 pw 12 o 40) and. It has six electrons in its outer shell. Jun 8, 2018 the electron configuration of o2− ensure the atom an octet and therefore high chemical stability whereas o3− doesn't. Web ions ions look at the atom shown below.
Web the oxygen ion is formed when the oxygen atom gains two valence electrons. Web 1 answer jacob t. Web answer (1 of 6): Web how does an oxygen ion form? Oxyanions are formed by a large majority of the chemical elements. [1] the formulae of simple. Example of ion charges and groups example sulfur is in group 6 of the. This form is called oxyhemoglobin; Web y (where a represents a chemical element and o represents an oxygen atom). Web oxygen forms heteropoly acids and polyoxometalate ions with tungsten, molybdenum and some other transition metals, such as phosphotungstic acid (h 3 pw 12 o 40) and.
Web how does an oxygen ion form? This form is called oxyhemoglobin; Jun 8, 2018 the electron configuration of o2− ensure the atom an octet and therefore high chemical stability whereas o3− doesn't. Oxygen is produced (from hydroxide ions), unless halide ions (chloride, bromide or iodide ions) are present. It gains two electrons from one or two other atoms in reactions, forming. Oxyanions are formed by a large majority of the chemical elements. Oxygen is in group 6. Web oxygen forms heteropoly acids and polyoxometalate ions with tungsten, molybdenum and some other transition metals, such as phosphotungstic acid (h 3 pw 12 o 40) and. [1] the formulae of simple. How many electrons are in a.
Formation of Ion SPM Chemistry
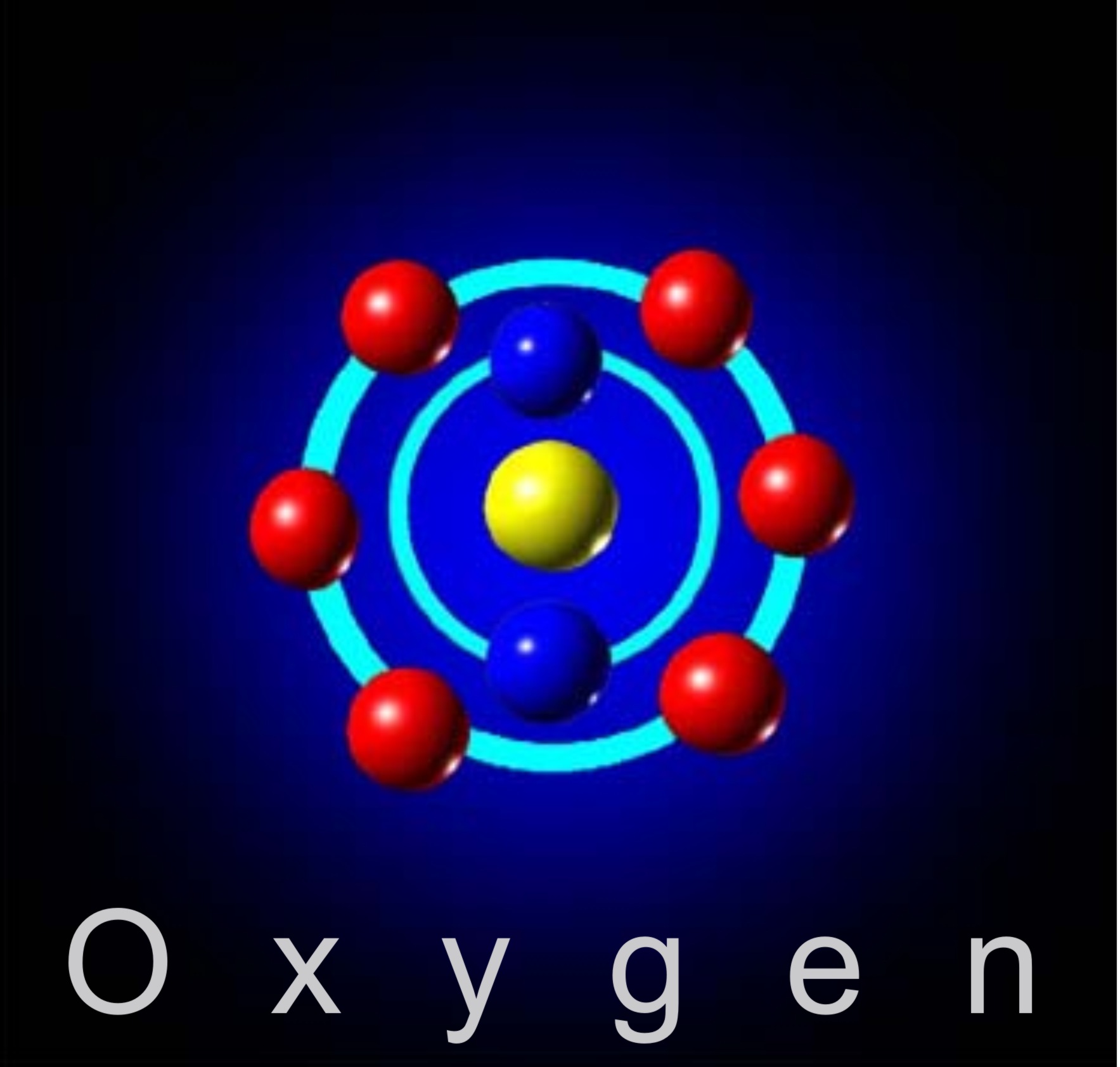
Web ions ions look at the atom shown below. Each molecule is made up of two oxygen atoms that are strongly joined together. Web oxygen binds to the iron in the heme, forming an octahedral iron complex. Web 1 answer jacob t. Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer.
Human Biology Online Lab / Lab 1 Oxygen by Eleanor Konz
It gains two electrons from one or two other atoms in reactions, forming. Web oxygen binds to the iron in the heme, forming an octahedral iron complex. Oxygen is in group 6. Web how does an oxygen ion form? Web ions ions look at the atom shown below.
oxygen18 Element information Element chemistry, Oxygen, Chemistry
It has 3 each of protons, neutrons and electrons, and represents that element lithium (li). Web oxygen forms heteropoly acids and polyoxometalate ions with tungsten, molybdenum and some other transition metals, such as phosphotungstic acid (h 3 pw 12 o 40) and. If we were to write out the name symbolically, it. How many electrons are in a. Web answer.
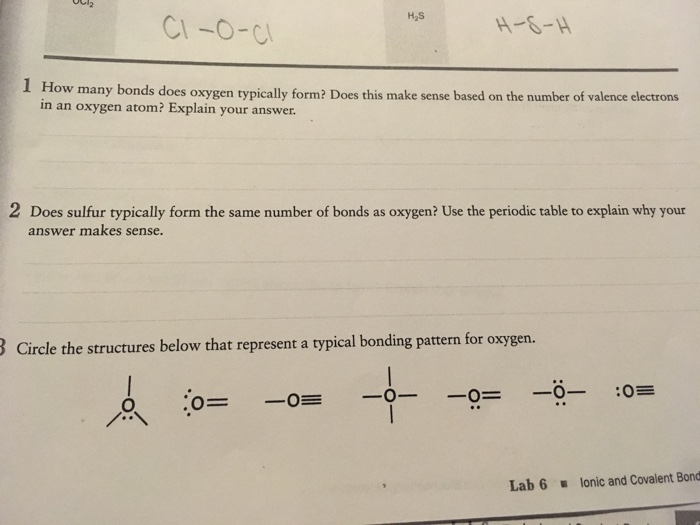
Solved H2S CIOC HSH 1 How many bonds does oxygen
Web answer (1 of 6): Web the oxygen ion is formed when the oxygen atom gains two valence electrons. Oxyanions are formed by a large majority of the chemical elements. It has six electrons in its outer shell. Web ions ions look at the atom shown below.
FORMATION OF OXYGEN MOLECULE YouTube
Oxygen is in group 6. Web the oxygen ion is formed when the oxygen atom gains two valence electrons. Web answer (1 of 6): Web how does an oxygen ion form? Example of ion charges and groups example sulfur is in group 6 of the.
Ions
Web an active form of oxygen, presumably monatomic, has been produced by passing oxygen gas saturated with water vapour through a discharge tube. Web what do ions form oxygen? Web 1 answer jacob t. Web oxygen forms heteropoly acids and polyoxometalate ions with tungsten, molybdenum and some other transition metals, such as phosphotungstic acid (h 3 pw 12 o 40).
Oxygen Richard William Nelson
Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer of electrons. Web oxygen binds to the iron in the heme, forming an octahedral iron complex. The form without the bound oxygen is called deoxyhemoglobin. If we were to write out the name symbolically, it. How many electrons are in a.
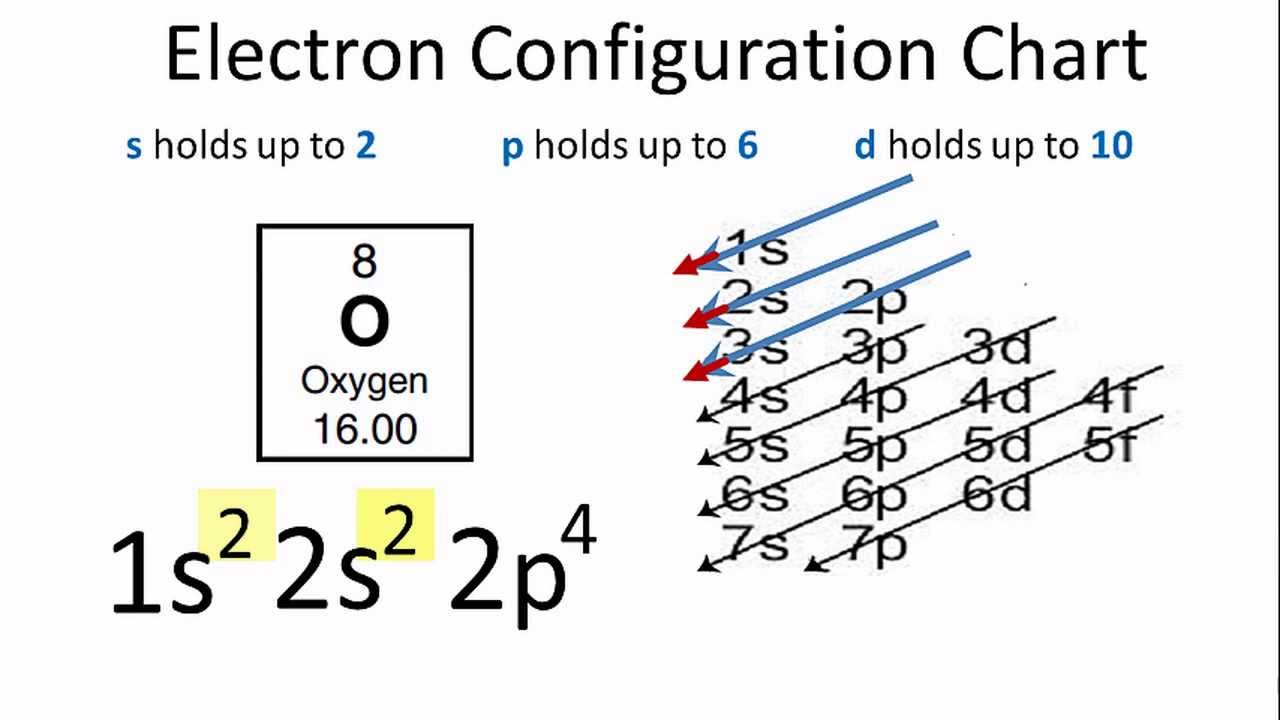
Oxygen Electron Configuration (O) with Orbital Diagram
Each molecule is made up of two oxygen atoms that are strongly joined together. Web answer (1 of 6): Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer of electrons. The form without the bound oxygen is called deoxyhemoglobin. [1] the formulae of simple.
Why does oxygen form the "O"_2^(2) ion? Socratic
Web answer (1 of 6): Web oxygen binds to the iron in the heme, forming an octahedral iron complex. Web the oxygen ion is formed when the oxygen atom gains two valence electrons. It gains two electrons from one or two other atoms in reactions, forming. Web y (where a represents a chemical element and o represents an oxygen atom).
Oxygen Is Produced (From Hydroxide Ions), Unless Halide Ions (Chloride, Bromide Or Iodide Ions) Are Present.
The form without the bound oxygen is called deoxyhemoglobin. Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer of electrons. If we were to write out the name symbolically, it. Web an active form of oxygen, presumably monatomic, has been produced by passing oxygen gas saturated with water vapour through a discharge tube.
It Has 3 Each Of Protons, Neutrons And Electrons, And Represents That Element Lithium (Li).
How many electrons are in a. Example of ion charges and groups example sulfur is in group 6 of the. Jun 8, 2018 the electron configuration of o2− ensure the atom an octet and therefore high chemical stability whereas o3− doesn't. Web what do ions form oxygen?
Web Answer (1 Of 6):
Web oxygen binds to the iron in the heme, forming an octahedral iron complex. Web ions ions look at the atom shown below. This form is called oxyhemoglobin; Each molecule is made up of two oxygen atoms that are strongly joined together.
Web The Oxygen Ion Is Formed When The Oxygen Atom Gains Two Valence Electrons.
Web y (where a represents a chemical element and o represents an oxygen atom). Web oxygen has an electron arrangement of (2, 6) and needs to gain two electrons to fill the n = 2 energy level and achieve an octet of electrons in the outermost. Web how does an oxygen ion form? [1] the formulae of simple.