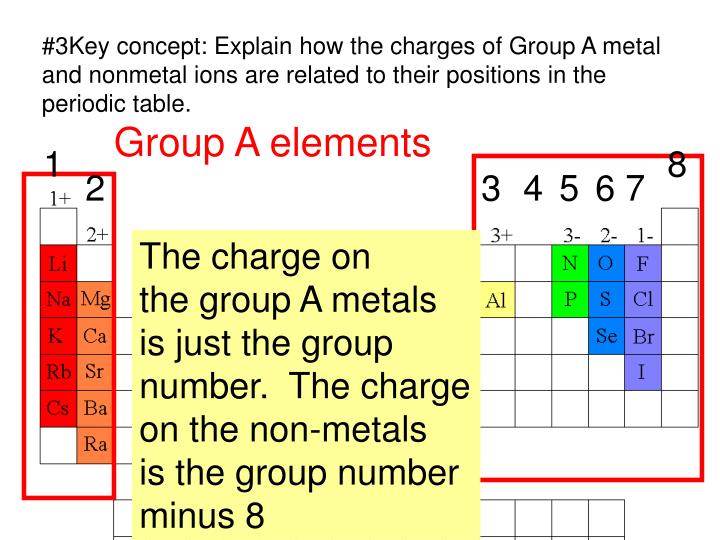
What Type Of Elements Form Anions
What Type Of Elements Form Anions - A cation has a net positive electrical charge, which means it has more protons than electrons. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Cations and anions to create an ion, atoms gain or lose electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Web anions are a type of atom, the smallest particle of an element that still retains the element's properties. Web 162k views types of ions: A sodium atom loses one electron to form a sodium ion forming negative ions Removal of the electron gives a cation (left), whereas the addition of an electron gives an. Web some common elements that form anions are hydrogen, fluorine, iodine and oxygen.
Web most of the elements that make ionic compounds form an ion that has a characteristic charge. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. An anion has a net negative. Web 162k views types of ions: Web there are two types of ions: A cation has a net positive electrical charge, which means it has more protons than electrons. Anions are so named because they are attracted to the anode (positive. Web forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web anions are a type of atom, the smallest particle of an element that still retains the element's properties. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases.
Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web anions and cations hydrogen atom (center) contains a single proton and a single electron. An anion has a net negative. Anions are so named because they are attracted to the anode (positive. A sodium atom loses one electron to form a sodium ion forming negative ions Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. 1, 2 and 3, the number of electrons lost is the same as the group number. These ions are negative because they contain more. Web some common elements that form anions are hydrogen, fluorine, iodine and oxygen. Web 162k views types of ions:
PPT IONS PowerPoint Presentation, free download ID2435906
Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not. Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. 1, 2 and 3, the number of electrons lost.
PPT Naming Compounds PowerPoint Presentation ID3350086
Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A sodium atom.
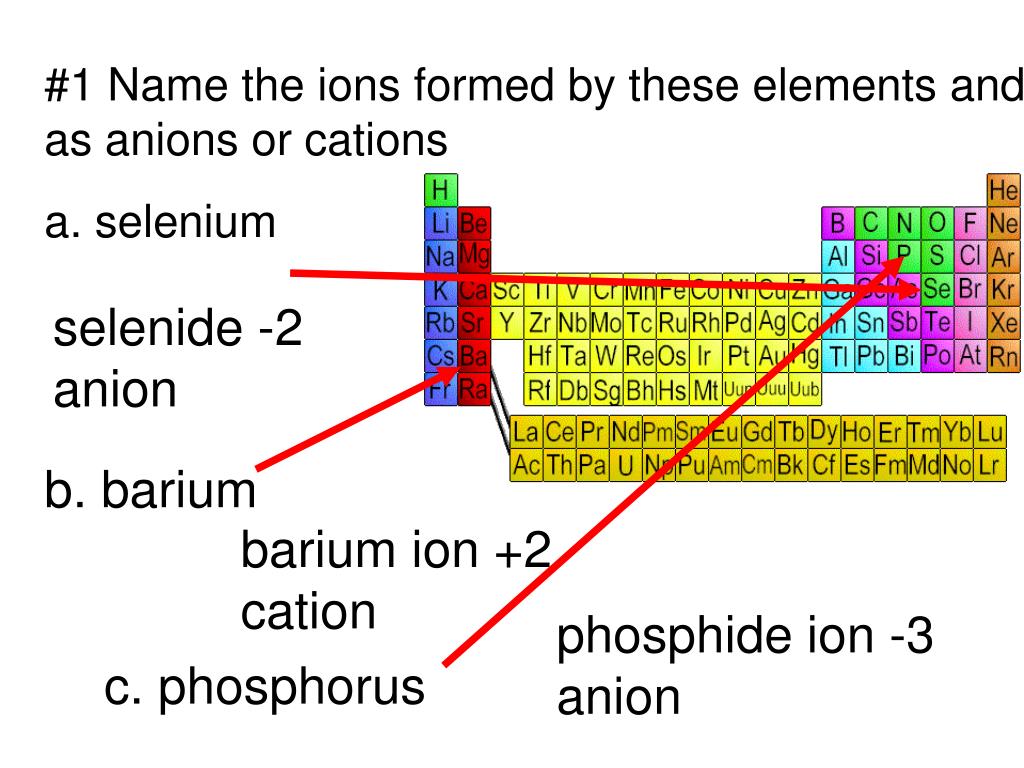
PPT 1 Name the ions formed by these elements and classify them as
1, 2 and 3, the number of electrons lost is the same as the group number. Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. A cation has a net positive electrical charge, which means it has more protons than electrons. Web atoms of group 17 gain one electron and form anions with.
PPT 1 Name the ions formed by these elements and classify them as
An anion has a net negative. Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Some atoms have nearly eight electrons in their valence shell and.
anion Common anions, their names, formulas and the elements they are
Removal of the electron gives a cation (left), whereas the addition of an electron gives an. Web 162k views types of ions: Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A sodium atom loses one electron to form a sodium ion forming negative ions Web anions are a type of atom,.
Image result for cations vs anions Chemistry classroom, Electron
Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web moving from the far right to the left on.
Naming Simple Ionic Compounds Pathways to Chemistry
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. For example, sodium makes ionic compounds in which the sodium. Web atoms of group 17 gain one electron and form anions with a 1− charge; Removal of the.
Which Group of Elements can Form Anions Most Quickly?
1, 2 and 3, the number of electrons lost is the same as the group number. Web there are two types of ions: Two types of ions can be created due to the gain and loss of electrons,. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. For example, sodium makes ionic.
The anion of an element has YouTube
Atoms are made of three types of subatomic particles: Anions are so named because they are attracted to the anode (positive. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web there are two types of ions: Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than.
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web atoms of group 17 gain one electron and form anions with a 1− charge; Web moving from the far right to the left on the periodic table, elements.
For Example, Sodium Makes Ionic Compounds In Which The Sodium.
Removal of the electron gives a cation (left), whereas the addition of an electron gives an. Two types of ions can be created due to the gain and loss of electrons,. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.
Web For Elements In Groups.
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Web there are two types of ions: Web most of the elements that make ionic compounds form an ion that has a characteristic charge. An anion has a net negative.
1, 2 And 3, The Number Of Electrons Lost Is The Same As The Group Number.
Atoms are made of three types of subatomic particles: Anions are negative ions that are formed when a nonmetal atom gains one or more electrons. Cations and anions to create an ion, atoms gain or lose electrons. Web negative ions, known as anions, form when an atom gains electrons and now has more electrons than protons, indicating that the number of protons and electrons is not.
Web Forming Negative Ions (Anions) Atoms Gain Electrons In Their Outer Shell When They Form Negative Ions, Called Anions.
Web 162k views types of ions: Anions are so named because they are attracted to the anode (positive. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web metals with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.