Which Combination Of Atoms Can Form A Polar Covalent Bond
Which Combination Of Atoms Can Form A Polar Covalent Bond - Web this creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the middle. Web advanced physics advanced physics questions and answers which combination of atoms can form a polar covalent bond? Web it takes two electrons to make a covalent bond, one from each bonding atom. The common factors that affect the conjugate base's stability are 1) the size and. A) h and br explanation: The atoms in this bond are xenon (electronegativity 2.6) and fluoride (electronegativity 4.0). This is due to one of the elements having a higher electronegativity than the. A h and h b h and f c n and n d na and f medium solution verified by toppr correct option is b) answer= h. Two atoms with equal electronegativity will. Web which combination of atoms can form a polar covalent bond?
Two atoms with equal electronegativity will. Lewis dot structures are one way to represent how atoms form covalent bonds. The polarity of a bond depends on the electronegativities of the bonded atoms. 2) ionic compounds where atoms are joined by ionic bond. The common factors that affect the conjugate base's stability are 1) the size and. Web a covalent bond is formed when two atoms share electron pairs. Web covalent bonds in which the sharing of the electron pair is unequal, with the electrons spending more time around the more nonmetallic atom, are called polar covalent. Let us consider a and b in which them is electronegativity difference. Web advanced physics advanced physics questions and answers which combination of atoms can form a polar covalent bond? Web non polar covalent bond is defined as type of chemical bond with equal sharing of the bond electron rises when the electronegativity of the two atoms are equal.
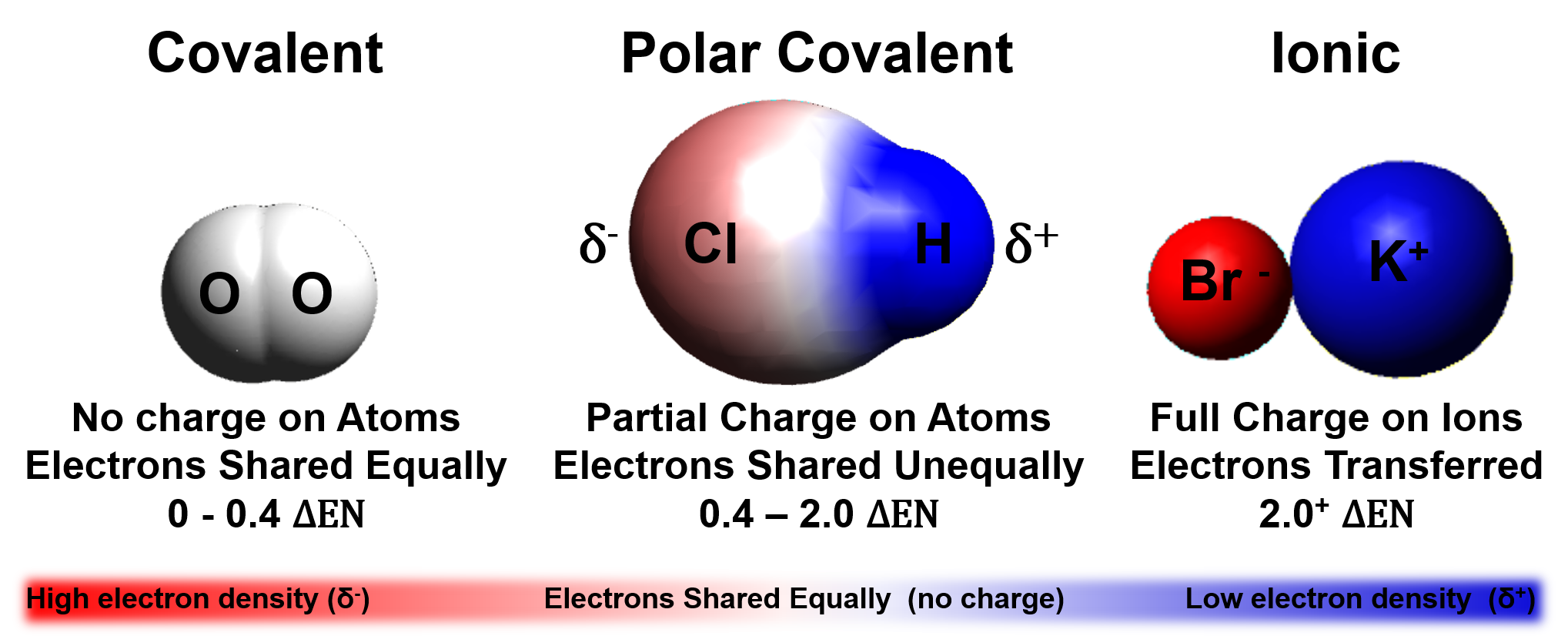
Web covalent bonds are also affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. This is due to one of the elements having a higher electronegativity than the. Web bonds between carbon and other elements such as oxygen and nitrogen are polar. Web non polar covalent bond is defined as type of chemical bond with equal sharing of the bond electron rises when the electronegativity of the two atoms are equal. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two. Web a covalent bond is formed when two atoms share electron pairs. Web this creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the middle. A h and h b h and f c n and n d na and f medium solution verified by toppr correct option is b) answer= h. Which molecule contains a non polar covalent bond? Web a polar covalent bond is a bond formed when a shared pair of electrons are not shared equally.
How does a polar bond differ from a covalent bond
Web the acid that forms the more stable conjugate base will be the stronger acid. Web it takes two electrons to make a covalent bond, one from each bonding atom. Which type of bond is present. Which molecule contains a non polar covalent bond? Web a polar covalent bond is a bond formed when a shared pair of electrons are.
Polar Covalent Bond Definitions, Types and Examples
Web polar covalent bonds are usually formed between two nonmetal atoms having different electronegativities. Web covalent bonds in which the sharing of the electron pair is unequal, with the electrons spending more time around the more nonmetallic atom, are called polar covalent. Which molecule contains a non polar covalent bond? Let us consider a and b in which them is.
What Is a Polar Bond? Definition and Examples
Two atoms with equal electronegativity will. Web what kind of bond is formed when two atoms share electrons to form a molecule? A h and h b h and f c n and n d na and f medium solution verified by toppr correct option is b) answer= h. Lewis dot structures are one way to represent how atoms form.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The atoms in this bond are xenon (electronegativity 2.6) and fluoride (electronegativity 4.0). Web this creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the middle. The polarity of a bond depends on the electronegativities of the bonded atoms. Two atoms with equal electronegativity will. Web the acid that forms.
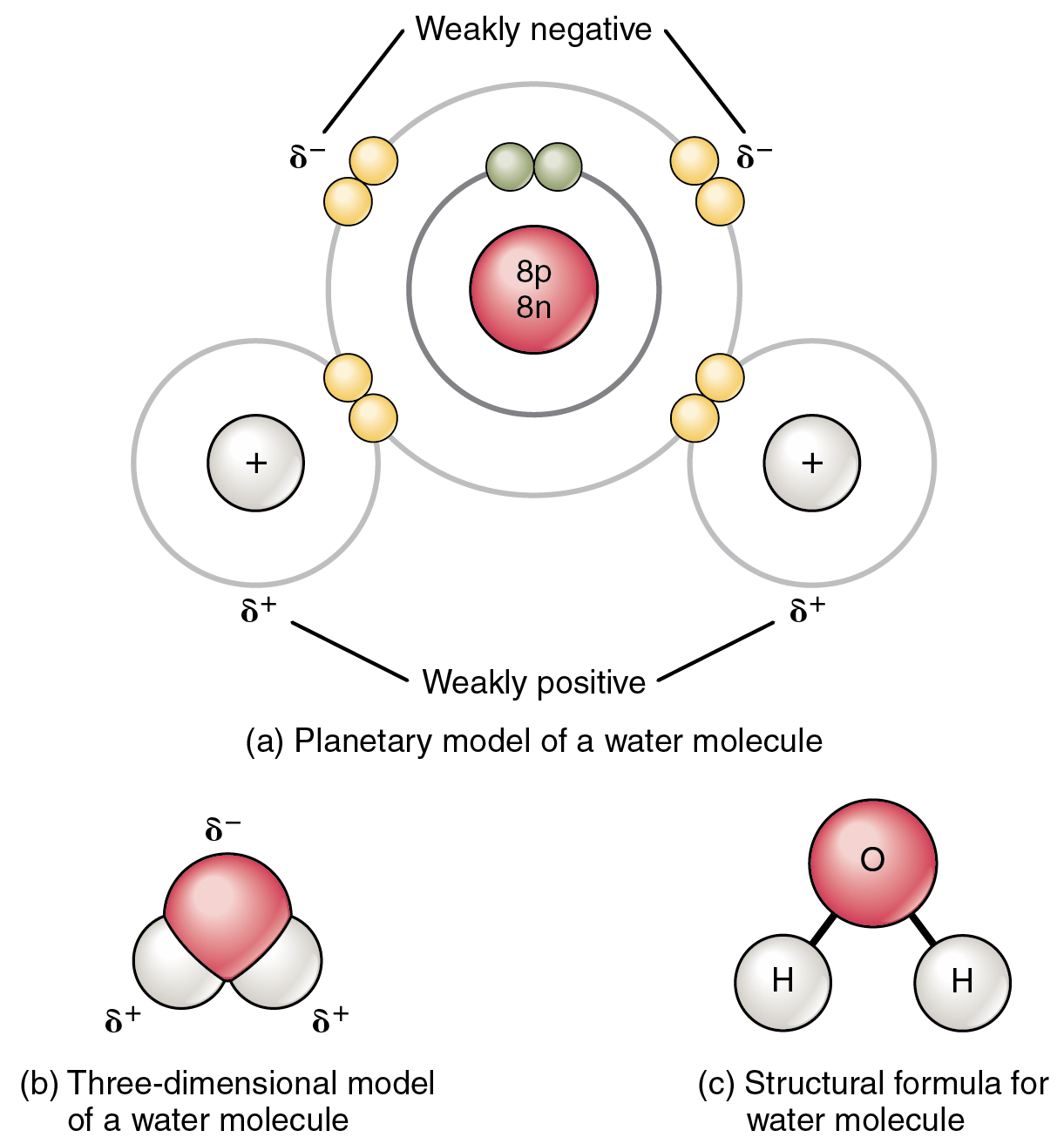
This figure shows the structure of a water molecule. The top panel
Web 1)molecular compounds where in atoms are joined by covalent bonds. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two. The common factors that affect the conjugate base's stability are 1) the size and. Let us consider a and b in which them is electronegativity difference. Only h and br form.
2.2 Chemical Bonds Anatomy & Physiology
A h and h b h and f c n and n d na and f medium solution verified by toppr correct option is b) answer= h. Web some compounds contain both covalent and ionic bonds. Two atoms with equal electronegativity will. The common factors that affect the conjugate base's stability are 1) the size and. Web which combination of.
Ch4 Polar Or Nonpolar Covalent Bond Which statement explains why a
2) ionic compounds where atoms are joined by ionic bond. The polarity of a bond depends on the electronegativities of the bonded atoms. Web some compounds contain both covalent and ionic bonds. Let us consider a and b in which them is electronegativity difference. Web which combination of atoms can form a polar covalent bond?
Building the World Be Prepared! Everything you should know for 1st
The common factors that affect the conjugate base's stability are 1) the size and. Web it takes two electrons to make a covalent bond, one from each bonding atom. Web bonds between carbon and other elements such as oxygen and nitrogen are polar. Let us consider a and b in which them is electronegativity difference. Web covalent bonds are also.
Covalent Bonds Biology for NonMajors I
A) hand br ob) hand h c) na and br od) n. Two atoms with equal electronegativity will. The polarity of a bond depends on the electronegativities of the bonded atoms. Web polar covalent bonds are usually formed between two nonmetal atoms having different electronegativities. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Web covalent bonds are also affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. Web some compounds contain both covalent and ionic bonds. A) hand br ob) hand h c) na and br od) n. Web the acid that forms the more stable conjugate base will be the stronger acid. Web polar covalent.
Web Some Compounds Contain Both Covalent And Ionic Bonds.
Lewis dot structures are one way to represent how atoms form covalent bonds. Web 1)molecular compounds where in atoms are joined by covalent bonds. Web what kind of bond is formed when two atoms share electrons to form a molecule? Web which combination of atoms can form a polar covalent bond?
Web The Acid That Forms The More Stable Conjugate Base Will Be The Stronger Acid.
Web it takes two electrons to make a covalent bond, one from each bonding atom. The common factors that affect the conjugate base's stability are 1) the size and. Web advanced physics advanced physics questions and answers which combination of atoms can form a polar covalent bond? 2) ionic compounds where atoms are joined by ionic bond.
This Is Due To One Of The Elements Having A Higher Electronegativity Than The.
Web a covalent bond is formed when two atoms share electron pairs. Web which combination of atoms can form a polar covalent bond? Web some compounds contain both covalent and ionic bonds. Web a polar covalent bond is a bond formed when a shared pair of electrons are not shared equally.
A H And H B H And F C N And N D Na And F Medium Solution Verified By Toppr Correct Option Is B) Answer= H.
A) hand br ob) hand h c) na and br od) n. Web covalent bonds are also affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. Let us consider a and b in which them is electronegativity difference. Web this creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the middle.
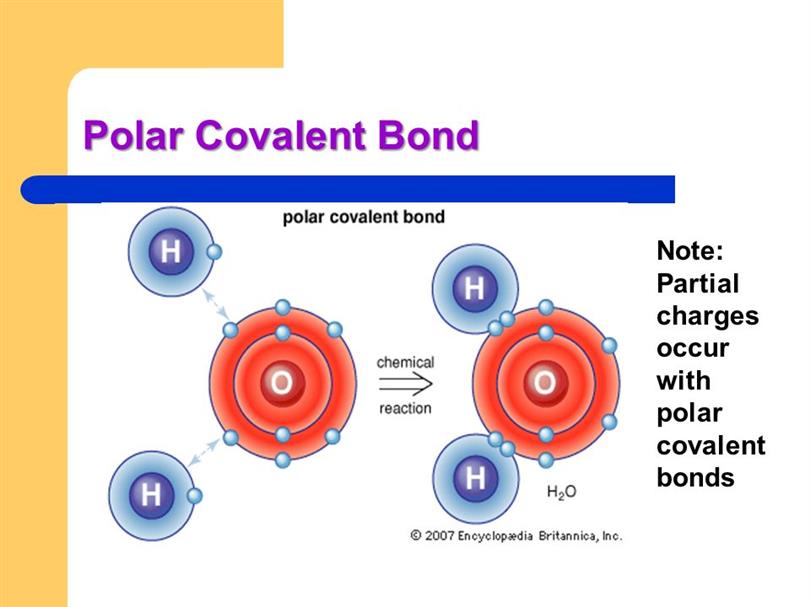
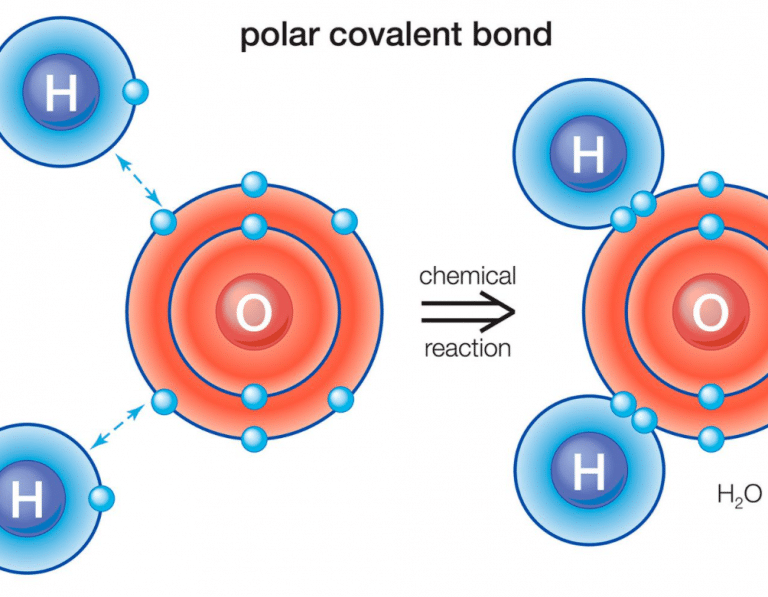
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)